Abstract
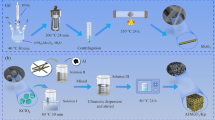
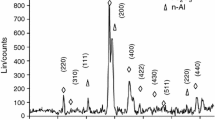
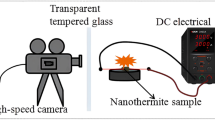
Following recent developments in micro-scale energy systems, such as microthruster and igniters among others, there is now considerable interest in using tertiary nanothermites to meet the increasing demand in high energy density propulsion systems. The first objective of this research is to compare and analyze the thermal behaviour of different nanothermite tertiary compositions based on nano-aluminium (n-Al), graphene oxide (GO), various salt and metallic oxidizers. The second objective is to identify the thermite reaction mechanism through correlations with the activation energy and exothermic peaks. Thermogravimetry analysis coupled with a differential scanning calorimeter (TGA/DSC) was employed to elucidate the reaction process of these nanothermite compositions, while bomb calorimetry was used to measure their heat of combustion. The apparent kinetics parameters were calculated using Kissinger and Ozawa approaches. The results demonstrate that the addition of GO enhances the reactivity of nanothermites with both salt and metallic oxidizers by reducing the reaction onset temperature, activation energy and increasing the heat release. For nanothermites with oxidizing salts, the heterogeneous solid–gas reaction mechanism plays a more important role than the condensed phase reactions. In general, nanothermites based on oxidizing salts are more reactive than those with metallic ones, as indicated in both theoretical and experimental data. Among them, the GO/Al/KClO4 nanothermite exhibits the highest heat release (9614 J g−1), while the GO/Al/K2S2O8 nanothermite shows the lowest onset temperature and activation energy (380 °C and 105 kJ mol−1). This study provides benchmark information for optimizing the tertiary nanothermites design, use, storage and handling.











Similar content being viewed by others
References
Trunov MA, Schoenitz M, Dreizin EL. Effect of polymorphic phase transformations in alumina layer on ignition of aluminium particles. Combust Theory Model. 2006;10(4):603–23.
Rai A, Park K, Zhou L, Zachariah MR. Understanding the mechanism of aluminum nanoparticle oxidation. Combust Theory Model. 2006;10(5):843–59.
Schoenitz M, Patel B, Agboh O, Dreizin EL. Oxidation of aluminum powders at high heating rates. Thermochim Acta. 2010;507–508:115–22.
Chowdhury S, Sullivan K, Piekiel N, Zhou L, Zachariah MR. Diffusive vs ex- plosive reaction at the nanoscale. J Phys Chem C. 2010;114(20):9191–5.
Levitas VI, Asay BW, Son SF, Pantoya M. Melt dispersion mechanism for fast reaction of nanothermites. Appl Phys Lett. 2006;89:071909.
LeSergent L., Tailoring the ignition and reaction properties of Cu2O thermite nanolaminates, In mechanical engineering-nanotechnology. 2018, Waterloo: Waterloo, Ontario, Canada. pp. 1–97
Kim SH, Zachariah MR. Enhancing the rate of energy release from nanoenergetic materials by electrostatically enhanced assembly. Adv Mater. 2004;16(20):1821–5.
Pantoya ML, Granier JJ. Combustion behavior of highly energetic thermites: nano versus micron composites. Propellants Explos Pyrotech. 2005;30(1):53–62.
Dreizin EL. Metal-based reactive nanomaterials. Prog Energy Combust Sci. 2009;35(2):141–67.
Jian G, Snehaunshu C, Sullivan K, et al. Nanothermite reactions: Is gas phase oxygen generation from the oxygen carrier an essential prerequisite to ignition? Combust Flame. 2013;160(2):432–7.
Wu C, K.S., Chowdhury S, Jian G, Zhou L and Zachariah MR, Encapsulation of Perchlorate Salts within Metal Oxides for Application as Nanoenergetic Oxidizers,. Adv Funct Mater. 2012; 22(1): 78–85.
Kappagantula KS, Farley C, Pantoya ML, Horn J. Tuning energetic material reactivity using surface functionalization of aluminum fuels. J Phys Chem C. 2012;116:24469–75.
Granier JJ, Pantoya ML. Laser ignition of nanocomposite thermites. Combust Flame. 2004;138:373–83.
Dean SW, Pantoya ML, Gash AE, Stacy SC, Hope-Weeks LJ. Enhanced convective heat transfer in nongas generating nanoparticle thermites. J Heat Transf. 2010;132:111201.
Wen JZ, Ringuette S, Bohlouli-Zanjani G, Hu A, Nguyen NH, Persic J, Pe-tre CF, Zhou YN. Characterization of thermochemical properties of Al nanoparti-cle and NiO nanowire composites. Nanoscale Res Lett. 2013;8:184.
Sullivan KT, Chiou WA, Fiore R, Zachariah MR. In situ microscopy of rapidly heated nano-Al and nano-Al/WO 3 thermites. Appl Phys Lett. 2010;97(13):133104.
Sullivan KT, Piekiel NW, Wu C, Chowdhury S, Kelly ST, Hufnagel TC, Fezzaa K, Zachariah MR. Reactive sintering: an important component in the combustion of nanocomposite thermites Combust. Flame. 2012;159(2):15.
Balandin AA, Ghosh S, Bao WZ, Calizo I, Teweldebrhan D, Miao F, Lau CN. Superior thermal conductivity of single layer graphene. Nano Lett. 2008;8:902–7.
Novoselov KS, Geim AK, Morozov SV, Jiang D, Katsnelson MI, Grigorieva IV, Dubonos SV, Firsov AA. Two-dimensional gas of massless diracfermions in graphene. Nature. 2005;438:197–200.
Stankovich S, Dikin DA, Dommett GHB, Kohlhaas KM, Zimney EJ, Stach EA, Piner RD, Nguyen ST, Ruoff RS. Graphene based composite materials. Nature. 2006;442:282–6.
Lee C, Wei XD, Kysar JW, Hone J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science. 2008;321:385–8.
Yan Q-L, Gozin M, Zhao F-Q, Cohena A, Pang S-P. Highly energetic compositions based on functionalized carbon nanomaterials. Royal Soc Chem Nanoscale. 2016;8:4799–851.
Ferrari AC, Bonaccorso F, Fal’ko V, et al. Science and technology roadmap for graphene, related two-dimensional crystals, and hybrid systems. Nanoscale. 2015;7:4598–810.
Thiruvengadathan R, Chung SW, Basuray S, Balasubramanian B, Staley CS, Gangopadhyay K, and Gangopadhyay S, A Versatile Self-Assembly Approach toward High Performance Nanoenergetic Composite Using Functionalized Graphene. Langmuir, American Chemical Society, 2014.
Yan N, Qin L, Hao H, Hui L, Zhao F, Feng H. Iron oxide/aluminum/graphene energetic nanocomposites synthesized by atomic layer deposition: Enhanced energy release and reduced electrostatic ignition hazard. Appl Surf Sci. 2017;408:51–9.
Fahd A, Dubois C, Chaouki J, Wen JZ, Youssef E. Synthesis and characterization of tertiary nanothermite CNMs/Al/KClO4 with enhanced combustion characteristics. Propellants Explos Pyrotech. 2021;46:995–1005.
Mokhtar MM, SAAEE, Hassaan MY, Morsy MS and Khalil MH, Thermally Reduced Graphene Oxide: Synthesis, Structural and Electrical Properties. Int J Nanoparticles Nanotech, 3:008, 2017. 3(1): p. 1–9.
Patil V, R.V.D., Rout TK, Banerjee S and Yadav GD Graphene oxide and functionalized multi walled carbon nanotubes as epoxy curing agents: a novelsynthetic approach to nanocomposites containing active nanostructured fillers, RSC Adv, 4: 49264–49272 (2014)
Peng-Gang Ren, D.-X.Y., Xu Ji, Tao Chen and Zhong-Ming Li (2011) Temperature dependence of graphene oxide reduced by hydrazine hydrate. Nanotechnology. 22: 1–8
Manoochehr Fathollahi HB. A comparative study of thermal behaviors and kinetics analysis of the pyrotechnic compositions containing Mg and Al. J Therm Anal Calorim. 2015;120:1483–92.
Yin K, Li H, Xia Y, Bi H, Sun J, Liu Z, Sun L. Thermodynamic and kinetic analysis of low-temperature thermal reduction of graphene oxide. Nano-Micro Lett. 2011;3(1):51–5.
Zhou W, DeLisio JB, Li X, Liu L, Zachariah MR. Persulfate salt as an oxidizer for biocidal energetic nano-thermites. J Mater Chem. 2015;3(22):11838–46.
Liu P, Wang T. Ultrasonic-assisted chemical oxidative cutting of multiwalled carbon nanotubes with ammonium persulfate in neutral media. Appl Phys A (Mater Sci Process). 2009;97:771–5.
Zhou W, De Lisio JBD, Wang X, Zachariah MR. Reaction mechanisms of potassium oxysalts based energetic composites. Combust Flame. 2017;177:1–9.
Oxley JC, Smith JL, Porter MM, Yekel MJ, Canaria JA. Potential Biocides: Iodine-Producing Pyrotechnics. Propell Explos Pyrot. 2017;42:1–18.
Pauling, L, General Chemistry, Dover Books on Chemistry. 2014.
Herbstein FH, Ron G, Weissman A, The thermal decomposition of potassium permanganate and related substances. Part I. Chemical aspects. J. Chem. Soc. A, 1971: 1821–1826.
Brown ME, Sole KC, Beck MW. Isothermal DSC study of the thermal decomposition of potassium permanganate. Thermochim Acta. 1985;89:27–37.
Becerra ME, Arias NP, Giraldo OH, López Suárez FE, Illán Gómez MJ, Bueno López A. Soot combustion manganese catalysts prepared by thermal decomposition of KMnO4. Appl Catal B Environ. 2011;102:260–6.
Wei C, JW, Pingyun L, Li L, Binhua C, Junjun D, Longxiang W, Yuan Y, Fengsheng L, Ignition and Combustion of Super-Reactive Thermites of AlMg/KMnO4. Rare Metal Mater Eng, 2013. 42(12): 2458–2461
Gunawana R, Freij S, Zhang D, Beach F, Littlefair M. Amechanistic study into the reactions of ammonium nitrate with pyrite. Chem Eng Sci. 2006;61:5781–90.
Sharma M, Sharma V, Effect of carbon nanotube addition on the thermite reaction in the Al/CuO energetic nanocomposite, in Philosophical Magazine 2017, Taylor & Francis Group. pp. 1921–1938
Yi W, Jiang W, Cheng Z, Chen W, An C, Thermite reactions of Al/Cu core-shell nanocomposites with WO3. Thermochimica Acta, 2007; 463: 69–76.
Shoemaker D.P., C.W.G., Steinfeld J.I., and Nibler J.W., Experiment 7, In Experiments in Physical Chemistry. 1981, McGraw-Hill: New York, NY. pp. 125–138
Fischer SH, G.M.C., A survey of combustible metals, thermites, and intermetallics for pyrotechnic applications, In 32nd AIAA/ASME/SAE/ASEE Joint Propulsion Conference, July 1996: Lake Buena Vista, Florida, USA
Grubelich SH, Grubelich MC, Theoretical energy release of thermites, intermetallics, and combustible metals, In 24th International Pyrotechnics Seminar. July 1998: Monterey, CA.
Farley CW, MLP, Losada M, and Chaudhuri S, Linking molecular level chemistry to macroscopic combustion behavior for nano-energetic materials with halogen containing oxides. J Chem Phys, 2013. 139: 1–8.
Farley C, Reactions of Aluminum with Halogen Containing Oxides, In Mechanical Engineering. May 2013, Texas Tech University: USA. p. 74.
Kissinger HE (1957) Reaction kinetics in differential thermal analysis. Anal Chem, 29(6): 1702.
Puszynski JA. Processing and characterization of aluminum-based nanothermites. J Therm Anal Calorim. 2009;96:677–85.
Song J, T.G., Yao M, Ding W, Zhang X, Bei F, Tang J, Huanga J and Yu Z, Thermal behavior and combustion of Al nanoparticles/MnO2-nanorods nanothermites with addition of potassium perchlorate. RSC Advances, 2019. 9: 41319–41325.
Jacob RJ, D.L.O.-M., Overdeep KR, Weihs TP, Zachariah MR, Incomplete reactions in nanothermite composites. J Appl Phys, 2017; 121: 054307.
Sullivan KT, W.-A.C., Fiore R, Zachariah MR, In situ microscopy of rapidly heated nano-Al and nano-Al/WO3 thermites. Appl Phys Lett, 2012; 97: 133104.
GC Egan, LaGrange T, Time-resolved nanosecond imaging of nanoscale condensed phase reaction. J Phys Chem, 2015; 119: 2792–2797.
Jacob RJ, Jian G, Guerieri PM, Zachariah MR. Energy release pathways in nanothermites follow through the condensed state. Combust Flame. 2015;162:258–64.
Sherif Elbasuney AF, Hosam E. Mostafa, Combustion characteristics of extruded double base propellant based on ammonium perchlorate/aluminum binary mixture. Fuel. 2017;208:296–304.
Elbasuney S, Gaber Zaky M, Radwan M, Mostafa SF. Stabilized super-thermite colloids: a new generation of advanced highly energetic materials. Appl Surf Sci. 2017;36:328–419.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fahd, A., Dubois, C., Chaouki, J. et al. Combustion behaviour and reaction kinetics of GO/Al/oxidizing salts ternary nanothermites. J Therm Anal Calorim 147, 10245–10257 (2022). https://doi.org/10.1007/s10973-022-11259-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-022-11259-x