Abstract
Results from our previous study proved that differential scanning calorimetry (DSC) is a reliable method for qualitative evaluation of morphological changes in human cartilage samples caused by cryopreservation and storage time correlates with graft quality [1, 2]. Our current aim was to specify/characterise the effects of deep-freezing on hyaline cartilage in conjunction with length of storage time. Detailed analysis included DSC and histological measurements which showed distinctive differences. Based on our histological examinations, we could not confirm significant association between cartilage destruction and long-time storage, but the DSC results exhibited a definite change in thermal parameters after the 6th week cryopreservation. A modified and more detailed analysis would be useful to determine the effects of deep-freezing.
Similar content being viewed by others
Introduction
Pathologic lesions affecting articular cartilage and subchondral bone such as traumatic focal osteochondritis dissecans (OCD) and patella chondromalacia mostly affect young and physically active age groups. Consequences in these conditions include progressive degeneration of articular cartilage (osteoarthritis), early retirement from an athletic career, and lower level of regular/daily physical activity. As a result, the risk of obesity and associated health problems such as cancers and early mortality increases [3]. Today it is still a common belief that hyaline cartilage has no or extremely limited natural regenerative potential, since the body cannot replace the lost/destroyed collagen leading to major loss of matrix and chondrocyte apoptosis at the site of the injury. Current surgical therapeutic approaches for cartilage repair to overcome cell and tissue deficiency are either reparative or restorative. The most frequently used method for the treatment of full-thickness defects of weight-bearing joints is the autologous osteochondral mosaicplasty developed by Hangody et al. [4]. The restoration of hyaline cartilage is based on the transfer of small osteochondral cylinders from the non-weight-bearing surface of the joint. The safety and reliability of this technique are related to low infection and tissue reaction risk, thus providing greater longevity, durability, and improved outcomes. Besides the advantages, it has known limitations due to a significant increase in donor-site morbidity and contour mismatch that must be taken into consideration and dealt with.
Due to organ donation, osteochondral allograft transplantation (OAC) has become a valid solution for the problems associated with autologous mosaicplasty. It also provides a viable alternative for total pain relief and functional recovery after cartilage restoration. Possible limitations of OAC that need further consideration and research are chronic or currently unknown, slow virus, and prion infections. Different storage procedures highly influence cell viability and immunogenicity of the graft, not to mention storage duration. A long storage time jeopardizes both biomechanical properties of the cartilage and viability of chondrocytes. In fresh allografts stored at 4–6 °C, signs of permanent damage to chondrocytes were already detectable after 21 days [5]. Even though deep-freezing of fresh-frozen allografts at –80 °C has the advantage of potential long storage time and decreased immunogenicity the adverse effect it has on chondrocyte survival is considerable.
Detailed analysis of structural and biomechanical changes and variations of cartilage collagen matrix, especially regarding differences in storage time, may provide valuable information on its possible applications for drug delivery, tissue regeneration, and engineering [6]. Our study focussed on OAC of traumatic articular injuries since secondary osteoarthritis (OA) is a substantial problem both from a personal and population perspective. Even though OA is a disease that is isolated to the cartilage of joints it can contribute to poor physical performance or even impairment not to mention its impact on mental health and psychosocial well-being. Research from past years started to focus on the magnitude and specification of psychological and mental issues associated with injury-related pain, consequent limitations in physical activity, and the emergence of more sedentary lifestyle. Anxiety and depression are interrelated with pain and physical limitation and can significantly impair QoL (quality of life) of patients by altering pain perception and functional capacity [7].
Even though progression is inevitable in these degenerative conditions, extensive research and clinical effort aimed to develop alternative approaches to halt or delay the onset of irreversible damage. Outcomes were not always satisfactory, and recently the main focus shifted towards the implementation and improvement of quality measurement and control.
Based on previous findings, DSC is a reliable method for the evaluation of the structural changes of cartilage samples and this way DSC analysis has clinical relevance in the quality assessment and performance improvement process of human allograft transplantation [1, 2, 8]. To enable a more thorough understanding regarding the biological and biomechanical effects of deep-freezing the primary objective of our current study was to explore the possibility and feasibility of other potential methods for impact evaluation and monitoring.
Histopathological methods were chosen since cartilage degeneration span through stages of destructive, micro- and macroscopic alterations/remodelling/transformations (mainly as a result of collagen degradation) and current histological staining is quite advanced and through chemical, molecular biology assays and immunological techniques the perspective of histochemical quantification of collagen seemed promising. Our intention was to find at least one or possibly even more potentially relevant method that strongly correlates with our DSC findings thus enable further research into its future applicability.
According to the latest findings, the utilization of histological analysis has not been studied so far, while other methods such as immunoassay, real-time PCR (polymerase chain reaction), etc. have already been considered and investigated [9].
Materials and methods
Sampling
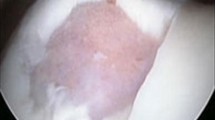
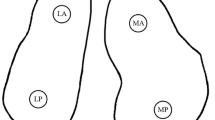
Human hyaline/articular cartilage samples were derived from femoral headpieces removed during hip hemi-prosthesis implantations in cases of fresh Garden III and IV type femoral neck fractures at the Department of Traumatology and Hand Surgery, University of Pécs, School of Medicine. These tissue fragments are considered as waste material to be disposed of. Donors were all females between the age of 65 and 75, without symptoms of osteoarthritis and have had arthroplasty within 12 h from injury. Samples were removed from the non-weight-bearing part of the femoral headpieces (see Fig. 1) and were fresh-frozen to be stored at −80 °C.
Cryopreservation times were: 0, 1, 3, 6, and 12 weeks. Fresh control samples were stored at + 4 °C in sterile 0.9% saline solution and were transported within 60 min to the DSC Laboratory at the Department of Biophysics, University of Pecs Medical School. To assess the change in medical and possible structural properties of the frozen human cartilage for further surgical use, we performed calorimetric examinations using differential scanning calorimetry (DSC).
DSC analysis
The freshly collected human cartilage samples have been prepared, measured within 6 h of removal, and kept at 4 °C during the whole process (‘controls’). Frozen samples stored at − 80 °C were thawed and processed using the same method as ‘controls.’ The thermal denaturation of different parts of human samples was monitored by a SETARAM Micro DSC-II calorimeter. All the experiments were performed between 0 and 100 °C. The heating rate was 0.3 K min−1. Conventional Hastelloy batch vessels with the average volume of 850 μL were used during the denaturation experiments. Typical sample wet masses for calorimetric experiments were in between 100 and 200 mg. Sterile physiologic saline solution was used as reference sample. Sample and reference vessels were equilibrated with a precision of ± 0.1 mg to eliminate the necessity of heat capacity correction. Calorimetric enthalpy was calculated from the area under the heat absorption curve by using two-point setting SETARAM peak integration. OriginPro 6.0 did the data treatment after ASCII conversion.
Histopathological examinations
Samples were processed and analysed at the Department of Pathology, University of Pecs Medical School based on the subsequent timeline: at the start, right after sample preparation (Trauma A), after 1 week (Trauma B), after 3 weeks (Trauma C), after 6 weeks (Trauma D), after 12 (Trauma E) and after 78 weeks (Trauma F) of deep-freezing (− 80 °C). After thawing standard protocol for formalin‐fixed paraffin-embedded tissue sample preparation was used (fixation with 10% neutral buffered formaldehyde followed by paraffin embedding). 4 µm sections were stained using haematoxylin–eosin, Hale colloidal iron and Masson’s trichrome stain. Haematoxylin–eosin staining (HE) was done using Leica ST 4040 linear staining system according to the following protocol: (1) deparaffination (3 × 2 min xylol then 3 × 2 min alcohol), (2) wash in tap water for 2 min, (3) stain sections in haematoxylin solution for 8 min, (4) wash in tap water for 8 min, (5) stain in eosin solution for 2 min, (6) rinse with alcohol 5 × 2 min, (7) rinse with xylol 3 × 2 min. Dry coverslipping was done using the fully automated Leica CV5030 coverslipper. For Hale colloidal iron stain deparaffinized and hydrated, unstained slides were pretreated with 12% acetic acid followed by the application and 60 min incubation with Müller colloidal iron working solution. After rinsing with 12% acetic acid for 3 × 2 min the visualization of iron was based on Prussian Blue-Forming Reaction. After bidistilled water washing, 1% neutral red nuclear stain was used. Sections were covered with a glass coverslip following tap-water rinsing. For Masson trichome staining deparaffinized and rehydrated tissue Sects. (1% acetic acid for 1 min followed by rinsing with tap water) were rinsed with the 1:2 mixture of scarlet acid fuchsin and Ponceau solution. After distilled water rinsing sections were treated with 1% phosphomolybdic acid solution for 5 min followed by a 2 min staining in aniline blue. After distilled water rinse and 1 min 1% acetic acid treatment the specimens were dehydrated in absolute alcohol. The procedure ended with dry coverslipping.
Histological evaluation included (1) the semiquantitative analysis of glycosaminoglycans (GAGs) using Hale colloidal iron reaction, (2) quantification of collagen using Masson trichome staining and (3) cell-density within the articular cartilage by HE.
Results and discussion
Thermal denaturation
Figure 2 and Table 1 show the run of DSC scans and the characteristic thermal parameters (Tms and ΔHcal, this later is normalised on wet sample mass) based on the thermal denaturation of cartilage samples which underwent cooling storage on − 80 °C with different time elapsed.
The most remarkable change caused by the freezing is a Tm around 85 °C after the first week storage compared to the control sample. The 3 weeks’ treatment resulted in a similar DSC scan as in case of control but the first two maxima shifted into the higher temperature range and a definite third one appeared around 77 °C. The effect of long-lasting cooling appeared after the 6th week: the first two Tms practically unchanged but the enthalpy contribution of these possible thermal domains changed oppositely; the first increased by twofold and the second decrease at least by same manner. We have finished the denaturation test after the 12th week, because the shape and the peak Tm changed (~ 10 °C) dramatically (see Fig. 2.), indicating dangerous change in the structure of cooled cartilage from the point of view for further surgery application.
Thermal data listed in Table 1 support the observed changes in Fig. 2. The Tms was read directly from the scans and the calorimetric enthalpy refers to the whole denaturation temperature range.
Up to the 6th week, we got higher denaturation temperatures and total calorimetric enthalpy than in case of control sample but from the 12th week, these parameters were decreased except for Tm2 and Tm3. We concluded that only samples stored frozen for six weeks could be proposed for further medical use to avoid complications.
Histological results
Since the detailed histological analysis has only been obtained on one sample so far, the results are not representative. The results on sample destruction in correlation to storage time are quite contradictory, as detailed below (Table 2).
We confirmed a 5–10% cell damage in the first 3 weeks using HE. After 6 weeks at − 80 °C, the rate of destruction was 50% which is consistent with the results of DSC. Somewhat contradictorily, rates of destruction were lower after longer periods of deep-freezing (≥ 12 weeks) than we expected. The reason for this inconsistency is unresolved.
We observed focal reductions in GAG after shorter periods of deep-freezing (1 and 3 weeks). The amounts of GAG seemed normal after longer storage time (≥ 6 weeks) which show no correlation with our DSC results.
Signs of collagen degradation were detectable after 1, 3 and 12 weeks of deep-freezing, although calculation of accurate degradation rate was not possible, and these findings did not correspond with DSC measurements.
Conclusions
Our current findings confirm the reliability and potential applicability of DSC for the quality assessment of human cartilage samples following longer preservation time [1, 2]. The potential relevance of histopathological analysis has been overestimated and did not meet the anticipated level of association with DSC scans, and these results do not show linearity. Based on the current outcome histological examinations are not suitable for the evaluation and monitoring of tissue destruction on the grounds of deep-freezing in the case of human articular cartilage. However, it is important to note that the detailed analysis has only been obtained on one sample so far, so the results are only referential and further evaluations are needed. Despite of the fact, that we have overestimated the relevance of histopathological analysis, appropriate data to confirm DSC results are expected from future electron microscopic and biochemical studies of the preserved cartilage, that are being planned/organized.
Data availability
There are no additional available data to upload.
References
Patczai B, Mintál T, Nőt LG, Wiegand N, Lőrinczy D. Effects of deep freezing and storage time on human femoral cartilage. J Thermal Anal Calorim. 2016;127(2):1177–80.
Patczai B, Sebestyen A, Wiegand N, Lőrinczy D. Structural evaluation of human cartilage after femoral neck fracture with differential scanning calorimeter. Nov Tech Arthritis Bone Res. 2017;1(3):555–63.
Jiang L, Rong J, Wang Y, Hu F, Bao C, Li X, et al. The relationship between body mass index and hip osteoarthritis: a systematic review and metaanalysis. Joint Bone Spine. 2011;78:150–5.
Hangody L, Füles P. Autologous osteochondral mosaicplasty for the treatment of full –thickness defects of weight-bearing joints: ten years of experimental and clinical experience. J Bone Joint Surg- Series A. 2003;85(1):25–32.
Malinin T, Temple HT, Buck BE. Transplantation of ostechondral allografts after cold storage. J Bone Joint Surg. 2006;88(4):762–70.
Musumeci G, Loreto C, Castorina S, Imbesi R, Leonardi R, Castrogiovanni P. Current concepts in the treatment of cartilage damage. A review. Ital J Anat Embryol. 2013;118:189–203.
Sharma A, Kudesia P, Shi Q, Gandhi R. Anxiety and depression in patients with osteoarthritis: impact and management challenges. Open Access Rheumatol. 2016;8:103–13.
Chae Y, Protsenko D, Lavernia EJ, Wong BJF. Effect of water content on enthalpic relaxations in porcine septal cartilage. J Thermal Anal Calorim. 2009;95:937.
Muinos-López E, Rendal-Vázquez ME, Hermida-Gómez T, Fuentes-Boquete I, Díaz-Prado S, Blanco FJ. Cryopreservation effect on proliferative and chondrogenic potential of human chrondrocytes isolated from superficial and cartilage. Open Orthop J. 2012;6:150–9.
Acknowledgements
This work was supported by CO-272 (OTKA) Grant (D.L.).
Funding
Open access funding provided by University of Pécs.
Author information
Authors and Affiliations
Contributions
IS: sample collection and handling, manuscript writing, BP: sample preparation and handling, manuscript writing, DL: corresponding author, principle investigator, DSC experiments, data analysis, manuscript writing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
All procedures followed were approved and in accordance with the ethical standards of the responsible committee on animal experimentation (institutional and national) and with the revised Helsinki Declaration of 1975.
Consent for publication
Copyright form has been uploaded with the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Szabó, I., Patzai, B. & Lőrinczy, D. Effects of long-term deep freezing on human femoral cartilage: differential scanning calorimetric (DSC) analysis and histopathological evaluations. J Therm Anal Calorim 147, 7793–7797 (2022). https://doi.org/10.1007/s10973-021-11070-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-021-11070-0