Abstract
The main goal of this study was to find out if thermal imaging may be useful in the evaluation of two types of anaesthetic injections—with and without a vasoconstrictor. There were 20 patients (13 women and 7 men) involved in the study. The group was divided into two subgroups. Patients from the first subgroup received infiltration anaesthesia (so-called: IA) of lidocaine (2 mL) and the second group included patients, who received infiltration anaesthesia (so-called: IAN) of lidocaine with 2% noradrenaline (2 mL). The obtained results showed a significant increase in the average temperature 10 min after the injection. In the IA group, the temperature increase was nearly 1.0 °C what was 0.3 °C higher than in the IAN group. Moreover, temperature changes showed a wide plateau between 10 and 25 min after anesthesia administration. The effect of temperature rise was also observed on the contralateral cheek where there was no intervention renders by a contralateral reflex.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Nowadays, good pain control is a standard in dental practice. At present, anesthetic agents are safe, effective, and can be applied with minimal risk of serious allergic reactions.
There is a basic classification of local anesthetics by their chemical substance—esters: cocaine, procaine, tetracaine, chlorocaine and amides: lidocaine, mepivacaine, prilocaine, bupivacaine, etidocaine, articaine, ropivacaine and levobupivacaine. The forms of the products available may vary from gels, ointments, sprays to solutions. Due to the duration of action, local anesthesia can be divided into short-acting ones, up to 30 min (2% lidocaine, 3% mepivacaine), intermediate duration up to 60 min (4% articaine, 2% lidocaine, 2% mepivacaine, 4% prilocaine, 2% procaine) and long-acting over 90 min (0.5% bupivacaine). We can distinguish vasodilating anesthesia such as lidocaine and articaine, as well as vasoconstrictive anesthesia such as cocaine, mepivacaine, ropivacaine (low doses) and additional substances added as vasoconstrictors: noradrenaline, adrenaline and felypressin [1].
There are topical anesthetics used on the surface of the skin and mucosa as well as injectable products. The common techniques include infiltration (field anesthesia) or conduction (nerve block) [2].
There are various techniques used in applying local anesthesia such as inferior alveolar block, long buccal block, mental nerve block, sublingual nerve block, posterior superior alveolar block, nasopalatine block, infraorbital block, infiltrations (supraperiosteally), intraligamentary anesthesia, electronic dental anesthesia (using the principles of transcutaneous electric nerve stimulation) and intraosseous anesthesia [3]. The daily dental practice is focused on achieving sufficient local anesthesia. Stimulation of nociceptors, responsive to noxious stimuli, results in pain. In general, sensory neuronal conduction of noxious stimuli is blocked by local anesthetics from accessing the central nervous system. [4]. The principle of local anesthesia is neuronal membrane stabilization through inhibition of ionic flow necessary for sending neural impulses. [2].
Permanently charged sodium channel blockers delivered through the pores of nociceptive ion channels can cause nociceptive neurons to be selectively blocked. Studies have shown that the majority of dental primary afferent neurons have nociceptive neuron features, such as capsaicin sensitivity and the presence of a hump in an action potential. As dental primary neurons express many nociceptor-specific ion channels and have the electrophysiological features of nociceptors, it may contribute to searching for a new way of entry for membrane-impermeable local anesthetic delivery [5].
Due to the mentioned vasodilative and vasoconstrictive features of the different types of anesthesia, it seems that their administration may lead to temperature changes in tissues.
Infrared thermal imaging is a scientific discipline based on infrared radiation detection from an object. The energy is conducted to the detector and processed to make a temperature distribution map, where temperature values are defined with a color scale [6]. Thermovision is commonly used in many applications, from industry to medicine. The several fields of medicine where thermal imaging is popular can be pointed, i.e., allergology, physiotherapy, oncology or even dentistry [7,8,9,10].
The relation between the tissue’s metabolism and temperature of the body allows using thermal imaging for diseases, injuries and inflammatory estimation. Monitoring the treatment and healing processes became easy to perform through temperature measurements [9, 11].
That is why for the purpose of this study, lesion-free areas were selected for injections and thermal imaging was performed to check the dynamics of thermal map changing due to the application of two anaesthetic injectables—lidocaine with or without noradrenaline for vasoconstriction.
Material and methods
A total of 20 cases took part in the study. The research group consisted of 13 women and 7 men between the age of 26 to 87 years. The characteristics of age and general oral cavity in both subgroups are shown below in Table 1. All patients, who took part in this study, were healthy without any ongoing infectious condition. Chronic conditions such as hypertension were controlled well. On examination patients reported no pain or other concerns. The oral cavity status was assessed by the dentist as good in both groups, which means that no acute conditions or abnormalities such as pulpitis or broken teeth were observed. No gingivitis, cysts or other conditions potentially correlated with inflammation were clinically diagnosed. Moreover, for every subject, the oral mucosa was examined, and in each case, the tissues were diagnosed as healthy.
The group was divided into two subgroups. Patients from the first subgroup received the infiltration anesthesia (so-called: IA) of lidocaine (2 mL) and the second group included patients, who received the infiltration anesthesia (so-called: IAN) of lidocaine with 2% noradrenaline (2 mL). Patients in both subgroups were given a single injection in various lesion-free areas—in one oral quarter. Moreover, no patient reported allergies to the administered anaesthetic.
Thermal imaging camera FLIR Systems T640 (FLIR Systems, Wilsonville, Oregon, United States) with a sensitivity of 0.03 K was used to perform measurements. The measurements were done in a Dental Clinic “Institute of Dentistry” in Katowice by two dentists. The climatic conditions in the measurement room were monitored and kept on a basic level of 20.0 ± 0.5 °C and humidity 46 ± 5%. Each patient had to adapt to the environment temperature for 20 min before the procedure and examination. According to the guidelines of thermal imaging diagnostics in medicine, during the adaptation process, patients stay calm in environmental conditions with uncovered imaged body regions [7, 10,11,12,13]. Patients were requested not to take painkillers or antipyretics in the last 24 h, antibiotics in the past week, consume alcohol within 24 h before the test, take sauna within 2 days prior to the test, and a potential physical exertion on the day of the test. The study group included only patients with 100% negative responses.
Each thermal imaging was carried out from a distance of about 0.7 ± 0.1 m from a patient and the camera was set perpendicularly to the measured region according to standards [11,12,13]. For imaging, the left and the right side of the face were chosen and set as an anesthesia side and a control side according to the procedure localization. Images were taken before the administration of anesthesia, directly after, and in 5, 10, 15, 20, 25 and 30 min after the injection. In each moment, imaging was done for both sides of the face (anesthesia and control side).
The research protocols were approved by the Bioethical Committee at the Silesian Medical Council (permission no. 37/2016).
The temperature parameters were calculated by averaging the selected areas shown in Fig. 1. The AR01 (large) ROI corresponded to the cheek area, and the AR02 (small) ROI indicated the anaesthesia localization (Fig. 1). The control side ROI matches with the anaesthesia ROI marked as AR02.
To ensure ROIs consistency between the subjects, the cheek area (AR01) was designated as a circle between the lobe of ear, the corner of the mouth and the corner of the eye for each patient. Moreover, the selected AR01 area could not overlap with the abovementioned structures. In case of anesthesia ROI (AR02), the localization of injection was taken into consideration, which was indicated by the dentist. The size of AR02 was chosen based on the visual assessment of temperature changes obtained on all thermal images for each patient.
The ThermaCAM TM Researcher Pro 2.8 SR-3 program and MS Office Excel 2019 were used to analyze achieved thermal images. The statistical analysis was performed with the t tests for dependent variables when the normality and homogeneity of variances were fulfilled and when not—Willcox’s tests. For physiology and temperature parameters, statistical analyses were made at a significance level of p < 0.05 on Statistica software. All significant results were marked on graphs.
Results
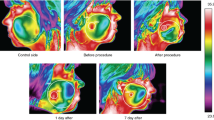
The obtained thermal images of anesthesia and the control side for both subgroups are shown in Figs. 2 and 3.
The primary visual evaluation of the thermal images presented in Fig. 2 shows slight temperature changes in contrast to Fig. 3 where one can see changes in temperature distribution directly after the procedure. An increase in temperature can be seen in the anesthesia area. On the other hand, a similar reaction seems to be visible on the control side of the face. This probably may indicate a contralateral reflex between body sides—in this study—faces. Moreover, the temperature changes are highly visible in Fig. 3 than 2 and on both sides of the face.
For a deeper analysis of the results for both groups, IA and IAN, the small area (AR02) statistical analysis was introduced and the temperature parameter taken into account was the average temperature. The results are shown in Fig. 4 for the IA group and in Fig. 5 for the IAN group.
In both groups, IA and IAN, the body surface (AR02—small) temperature tends to increase. However, in the IA group, the temperature change is almost 1.0 °C (Fig. 4). In the IAN group, the temperature tends to rise up, however, the difference is not as high as obtained for the IA group and it is about 0.7 °C (Fig. 5). In both groups, the temperature rising trend can be observed until 10 min after the procedure, although it is clearly visible for the IA group. After this time one can see some wide plateau period with small fluctuations observed between 10 and 25 min after anesthesia administered in both groups. Moreover, higher SD values can be noticed in the group IAN (Fig. 5).
It can be also seen that for the IA group (Fig. 4) the temperature raised statistically significantly after 10 min of the procedure and level p < 0.05 has been achieved.
Figure 6 presents the average temperature changes of the control cheek. Area, which was used to calculate the average temperature on the control side, corresponds to the AR02 located on the treatment side.
One can see in Fig. 6 the significant increase of the average temperature for the control side obtained and marked in Fig. 6 in time before and 15 min after and before and 20 min after the procedure. It should be also noted that the temperature difference observed in Fig. 6 is almost 0.7 °C. Such a magnitude of changes in the average temperature on the control side may be related to the contralateral reflex.
However, for the IAN group, the temperature changes in time for the control side are lower and not statistically significant than which is presented in Fig. 7.
Discussion
Although the procedure of anesthesia was administered to the area between mucosa and gingiva, a little decrease of the cheek temperature was expected due to the lower temperature of the injected product and a vasoconstrictor added. However, the observed reaction was reverse and seen on both sides, with a lower temperature change of the control cheek. Obtained temperature changes are clearly seen in thermal images presented in Figs. 2 and 3. Such results may be associated with changes in blood flow in cheek tissue due to interference caused by the anesthetic agent. It may suggest that some effects of the procedure performed in the oral cavity reflects also on the cheek surface. Moreover, temperature changes observed on the cheek showed some tendency to increase and this effect has been obtained in both subgroups (IA and IAN) for AR02 ROI’s.
However, it should be underlined that body surface temperature relates to many external and internal factors, which may have impact to the temperature results. Some physiological reactions may also be correlated with the stress level [10, 14,15,16]. Moreover, intervention in soft tissue (in this study it was gingiva and mucosa) may also cause the reaction of superficial tissue being in touch with the place of the intervention and its closest surroundings which was reported previously [10, 17, 18].
The observations seen in thermal images have been confirmed by average temperature changes in time observed in anesthesia regions for both groups (Figs. 4 and 5) which may suggest the inducing inflammation due to the injection of the external chemical substance. Such effects can be easily traced by thermovision [10, 17,18,19].
It should be noted that the main goal of the study was to show immediate and time changes in thermal reaction of the body on two types of dental anaesthesia procedures. It was mainly dictated by dentist’s practice and patient’s feelings. It is known that anaesthetic drugs injected to the tissue lead to vasoconstriction, so it seems to result in lowering the temperature. However, in provided studies, only the body surface was imaged not the gingiva. Obtained results showed the temperature increase of both sides of mouth and some repeatable temperature changes such as a wide plateau between 10 and 25 min after anesthesia administration. Such observation and the time of the plateau as well as the magnitude of temperature increase after anaesthesia administration may bring some quantitative thermal parameters which may correlated with patients’ feelings and prognostic of the possible side effects (i.e., time of pain sensation for patients) after treatment procedure. Moreover, obtained results clearly confirmed the contralateral effect observed after anaesthetic procedures which seems to be an important physiological observation. On the other hand, there were obtained differences in thermal body response between two used anaesthesia procedures. In the IA group, the temperature increase was nearly 1.0 °C and it was 0.3 °C higher than in the IAN group, most probably due to lack of vasoconstriction in IA group. Such effect was not observed since now and may come out of difference in reaction on those two drugs. In our opinion, it may be interesting to widen the studies.
Assessing temperature changes by dentists can be an important diagnostic tool to exclude inflammation processes not known by patients, previous medical and dental treatments (i.e., Dermal fillers) not reported by patients when taking medical history or when full verbal examination is not possible due to learning disabilities, dementia or other mental health conditions. Immediate temperature assessment can give information on changes in circulation such as vessel obstruction due to pressure, increased vasoconstriction with a significant decrease in temperature or sudden increase in temperature due to an allergic reaction.
Observing distribution of an injected fluid with anesthetic agent in thermal imaging can help assess the correct administration of infiltration anesthesia as well as monitor correlation with patient’s sensations.
The observed temperature rise is probably similar to the tissue inflammatory reaction during surgical intervention. In this case, lidocaine, which provides slight vasodilatation [1], and noradrenaline, which provides vasoconstriction [1], have been administered in anesthesia which may lead to an increase and a decrease of soft tissue temperature [20]. The pressure from the administered solution may stimulate mechanoreceptors leading to pain reduction. Plateau temperature changes between 10 and 25 min after anesthesia can be observed, as anesthesia achieves homogeneity, and it may be correlated with distending of the solution in the tissue and reaction similar to the inflammatory state. It may indirectly confirm that cheek temperature changes are correlated with some inflammation appearing after the injection—minor external intervention which has been reported in surgical teeth extraction [10].
The temperature increase in the control side of the face may be a result of the contralateral reflex (Fig. 6). The temperature is increasing, although the difference is lower than for the treatment side (Fig. 4). Evidently, the contralateral reflex is weaker than the original reaction and it is about 60% of primary response and such effect is observed in the previously performed studies [21].
Conclusions
A significant increase in the average temperature 10 min after injection on the cheek, where intervention was performed, was obtained. Moreover, in the IA group, the temperature increase was nearly 1.0 °C and it was 0.3 °C higher than in the IAN group. The analysis of temperature parameters changes with time showed a wide plateau between 10 and 25 min after anesthesia was administered. The effect of the temperature increase was also observed on the contralateral cheek where there was no intervention what seems to be caused by the contralateral reflex.
References
Dominiak M, Zapała J, Gedrange T. Podstawy chirurgii stomatologicznej. Wrocław: Elsevier Urban & Partner; 2013.
Ogle OE, Mahjoubi G. Local anesthesia: agents, techniques, and complications. Dent Clin. 2012;56(1):133–48.
Da Silva JD, Mitchell DA, Mitchell L, Brunton P. Oxford American handbook of clinical dentistry. Oxford: Oxford University Press; 2008.
Bahl R. Local anesthesia in dentistry. Anesth Prog. 2004;51(4):138.
Kim HY, Chung G, Jo HJ, Kim YS, Bae YC, Jung SJ, Kim JS, Oh SB. Characterization of dental nociceptive neurons. J Dent Res. 2011;90(6):771–6.
Więcek B, De Mey G. Termowizja w podczerwieni, podstawy i zastosowania. Warsaw: Wydawnictwo PAK: Warszawa; 2011.
Minkina W. Pomiary termowizyjne – przyrządy i metody. Częstochowa: Wydawnictwo Politechniki Częstochowskiej; 2004.
Cholewka A, Kasprzyk T, Stanek A, Sieroń-Stołtny K, Drzazga Z. May thermal imaging be useful in cyclist endurance tests? J Therm Anal Calorim. 2015. https://doi.org/10.1007/s1097301546625.
Boerner E, Podbielska H. Application of thermal imaging to assess the superficial skin temperature distribution after local cryotherapy and ultrasound. J Therm Anal Calorim. 2017. https://doi.org/10.1007/s1097301767728.
Kasprzyk-Kucewicz T, Cholewka A, Bałamut K, Kownacki P, Kaszuba N, Kaszuba M, Stanek A, Sieroń K, Stransky J, Pasz A, Morawiec T. The applications of infrared thermography in surgical removal of retained teeth effects assessment. J Therm Anal Calorim. 2019. https://doi.org/10.1007/s1097302009457-6.
Bauer J, Dereń E. Standaryzacja badań termograficznych w medycynie i fizykoterapii. Acta Bio-Opt Inform Med. 2014;20:11–20.
Ammer K. The Glamorgan Protocol for recording and evaluation of thermal images of the human body. Thermol Int. 2008;18:125–9.
Ammer K, Ring F. The thermal human body, A practical guide to thermal imaging. New York: Jenny Stanford Publishing; 2019.
Fita K, Dobrzyński M, Całkosiński I, Dudek K, Bader-Orłowska D. Przydatność termografii w diagnostyce lekarsko-stomatologicznej – doświadczenia własne. Ann Acad Med Stetin. 2007;53:34–8.
Herman IP. Physics of the Human Body. New York: Springer; 2016.
Charkoudian N. Skin blood flow in adult human thermoregulation: how it works, when it does not, and why. Mayo Clin Proc. 2003. https://doi.org/10.4065/78.5.603.
Kasprzyk-Kucewicz T, Cholewka A, Kaszuba M, Kaszuba N, Stanek A, Sieroń K, Morawiec T. The applications of thermal imaging in dental implants—pilot study. Inżynier Fizyk Medyczny. 2019;6:479–83.
Kasprzyk-Kucewicz T, Bałamut K, Kaszuba M, Kaszuba N, Stanek A, Sieroń K, Kopczyńska E, Cholewka A, Morawiec T. The applications of thermal imaging in dentistry—pilot study. Inżynier Fizyk Medyczny. 2018;2:130–2.
Madura H. Pomiary Termowizyjne w praktyce. Warszawa: Agenda Wydawnicza PAKu; 2004.
Dominiak M. Podstawy chirurgii stomatologicznej. Wrocław: Akademia Medyczna im. Piastów Śląskich; 2010.
Isii Y, Matsukawa K, Tsuchimochi H, Nakamoto T. Ice-water hand immersion causes a reflex decrease in skin temperature in the contralateral hand. J Physiol Sci. 2007;57(4):241–8.
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
Conceptualization: A.C., A.S.D.W.-K.; Methodology: K.B., N.K., T.K.-K.; Formal analysis and investigation: N.K., T.K.-K., A.C.; Writing—original draft preparation: N.K.; Writing—review and editing: T.M.; Supervision: A.C., T.M.
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethics approval
The research protocols were approved by the Bioethical Committee at the Silesian Medical Council (Permission No. 37/2016).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kaszuba, N., Kasprzyk-Kucewicz, T., Bałamut, K. et al. May thermal imaging be useful in the assessment of dental anaesthesia? Preliminary study. J Therm Anal Calorim 147, 6745–6753 (2022). https://doi.org/10.1007/s10973-021-10985-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-021-10985-y