Abstract
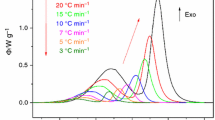
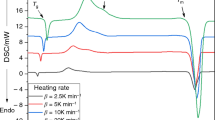
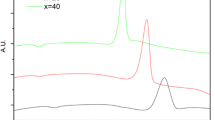
The particle size effects on the kinetic parameters of two overlapping crystallization peaks of Se85Te10Sb5 chalcogenide glass were studied using differential scanning calorimetry (DSC) under experimental and predicted isothermal conditions. The crystallization kinetics parameters of the two peaks were separated based on the application of a multi-peak Gaussian function using the advanced thermokinetics software package (AKTS). The nonisothermal methods of Friedman, Kissinger–Akahira–Sunose and the minimization, in addition to the predicted isothermal method, were used to investigate the variation of the effective activation energy with the extent of crystallization and, hence, with temperature. The local Avrami exponent as a function of the crystalline volume fraction was obtained at a constant heating rate or temperature for nonisothermal and isothermal processes. Its value was found to vary with particle size. The crystallization process of the two peaks was found to follow the Avrami–Erofeev reaction model.














Similar content being viewed by others
References
Ganjoo A, Jain H, Yu C, Song R, Ryan JV, Irudayaraj J, Ding YJ, Pantano CG. Planar chalcogenide glass waveguides for IR evanescent wave sensors. J Non-Cryst Solids. 2006;352:584–8.
Harbold JM, Ilday FO, Wise FW, Aitken BG. Highly nonlinear Ge–As–Se and Ge–As–S–Se glasses for all-optical switching. IEEE Photon Technol Lett. 2002;14:822–4.
Joraid AA. Estimating the activation energy for the non-isothermal crystallization of an amorphous Sb9.1Te20.1Se70.8 alloy. Thermochim Acta. 2007;456:1–6.
Joraid AA, Abu-Sehly AA, Alamri SN. A study on isothermal kinetics of glassy Sb9.1Te20.1Se70.8 alloy. J Taibah Univ Sci. 2009;2:106–17.
Abdel-Rahim MA, Hammam Mohamed AS, Abu-Sehly AA, Hafiz MM. Composition effect on the pre-crystallization and crystallization characteristics for Se90-xTe10Agx. J Alloys Compd. 2017;728:1346–61.
Yin H, Li L, Liu Y, Hu L, Zeng H, Chen G. Impact of tellurium on glass transition and crystallization in the Ge-Se-Te-Bi system. Ceram Int. 2017;43:3556–61.
Abdel-Rahimn MA, Hafiz MM, Mahmoud AZ. Crystallization kinetics of over lapping phases in Se70Te15Sb15 using isoconversional methods. Prog Nat Sci Mater Int. 2015;25:169–77.
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520:1–19.
Vyazovkin S, Chrissafis KM, DiLorenzo L, Koga N, Pijolat M, Roduit B, Sbirrazzuoli N, Suñol JJ. ICTAC Kinetics Committee recommendations for collecting experimental thermal analysis data for kinetic computations. Thermochim Acta. 2014;590:1–23.
Farjas J, Roura P. Isoconversional analysis of solid-state transformations. A critical review. Part I. Single step transformations with constant activation energy. J Therm Anal Calorim. 2011;105:757–66.
Farjas J, Roura P. Isoconversional analysis of solid state transformations. A critical review. Part II Complex transformations. J Therm Anal Calorim. 2011;105:767–73.
Farjas J, Roura P. Isoconversional analysis of solid-state transformations. A critical review. Part III. Isothermal and nonisothermal predictions. J Therm Anal Calorim. 2012;109:183–91.
Joraid AA, Abu El-Oyoun M, Afify N. Phase separation and crystallization kinetics studies of amorphous Si10Te90. Chalcogenide Lett. 2016;13:79–89.
Joraid AA, Okasha RM, Al-Maghrabi MA, Afifi TH, Agatemor C, Abd-El-Aziz AS. Thermal degradation behavior of a new family of organometallic dendrimer. J Inorg Organomet Polym Mater. 2020. https://doi.org/10.1007/s10904-020-01444-6.
Friedman HL. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J Polym Sci C. 1964;6:183–95.
Kissinger HE. Variation of peak temperature with heating rate in differential thermal analysis. J Res Nat Bur Stand. 1956;57:217–21.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Akahira T, Sunose T. Method of determining activation deterioration constant of electric insulating materials. Res Rep Chiba Inst (Sci Technol). 1971;16:22–31.
Vyazovkin S. Evaluation of activation energy of thermally stimulated solid-state reactions under arbitrary variation of temperature. J Comput Chem. 1997;18:393–402.
Vyazovkin S. Modification of the integral isoconversional method to account for variation in the activation energy. J Comput Chem. 2001;22:178–83.
Vyazovkin S. Isoconversional kinetics of thermally stimulated processes. Springer Int Publ Switzerland. 2015. https://doi.org/10.1007/978-3-319-14175-6.
Joraid AA, Abu-Sehly AA, Alamri SN. Isoconversional kinetic analysis of the crystallization phases of amorphous selenium thin films. Thin Solid Films. 2009;517:6137–41.
Ray CS, Day DE. Identifying internal and surface crystallization by differential thermal analysis for the glass-to-crystal transformations. Thermochim Acta. 1996;280(281):163–74.
Ray CS, Day DE, Huang W, Narayan KL, Kelton KF. Non-isothermal calorimetric studies of the crystallization of lithium disilicate glass. J Non-Cryst Solids. 1996;204:1–12.
Bednarcik J, Burkel E, Saksl K, Kollar P, Roth S. Mechanically induced crystallization of an amorphous CoFeZrB alloy. J Appl Phys. 2006;100:014903.
Pustkova P, Zmrhalova Z, Málek J. The particle size influence on crystallization kinetics of (GeS2)01(Sb2S3)09 glass. Thermochim Acta. 2007;466:13–21.
Svoboda R, Malek J. Interpretation of crystallization kinetics results provided by DSC. Thermochim Acta. 2011;526:237–51.
Joraid AA. Limitation of the Johnson-Mehl-Avrami (JMA) formula for kinetic analysis of the crystallization of a chalcogenide glass. Thermochim Acta. 2005;436:78–82.
Joraid AA. The effect of temperature on nonisothermal crystallization kinetics and surface structure of selenium thin films. Phys B. 2007;390:263–9.
Sestak J, Simon P (Editors). Thermal analysis of micro, nano- and non-crystalline materials -transformation, crystallization, kinetics and thermodynamics. Springer, Heidelberg. DOI https://doi.org/10.1007/978-90-481-3150-1
International Centre for Diffraction Data (ICDD). PDF-4+ 2018.
Roduit B. Prediction of the progress of solid-state reactions under different temperature modes. Thermochim Acta. 2002;388:377–87.
Burnham AK, Dinh LN. A comparison of isoconversional and model-fitting approaches to kinetic parameter estimation and application prediction. J Therm Anal Calorim. 2007;89:479–90.
Roduit B, Xia L, Folly P, Berger B, Mathieu J, Sarbach A, Andres H, Ramin M, Vogelsanger B, Spitzer D, Moulard H, Dilhan D. The simulation of the thermal behavior of energetic materials based on DSC and HFC signals. J Therm Anal Calorim. 2008;93:143–52.
Roduit B, Dermaut W, Lunghi A, Folly P, Berger B, Sarbach A. Advanced kinetics-based simulation of time to maximum rate under adiabatic conditions. J Therm Anal Calorim. 2008;93:163–73.
Joraid AA, Alhosuini IMA. Effect of heating rate on the kinetics and mechanism of crystallization in amorphous Se85Te10Pb5 glasses. Thermochim Acta. 2014;595:28–34.
Vyazovkin S, Wight CA. Isothermal and nonisothermal reaction kinetics in solids: In search of ways toward consensus. J Phys Chem A. 1997;101:8279–84.
Turnbull D, Fisher JC. Rate of nucleation in condensed systems. J Chem Phys. 1949;17:71–3.
Majumdar S, Sharma IG, Bidaye AC, Suri AKA. study on isothermal kinetics of thermal decomposition of cobalt oxalate to cobalt. Thermochim Acta. 2008;473:45–9.
Blazquez JS, Conde CF, Conde A. Non-isothermal approach to isokinetic crystallization processes: application to the nanocrystallization of HITPERM alloys. Acta Mater. 2005;53:2305–11.
Blazquez JS, Conde CF, Conde A, Kulik T. A direct extension of the Avrami equation to describe the non-isothermal crystallization of Al-base alloys. J Alloys Compd. 2007;434–435:187–9.
Ramasamy P, Stoica MA, Taghvaei H, Prashanth KG, Kumar R, Eckert J. Kinetic analysis of the non-isothermal crystallization process, magnetic and mechanical properties of FeCoBSiNb and FeCoBSiNbCu bulk metallic glasses. J Appl Phys. 2016;119:073908.
Rahvard MM, Tamizifar M, Boutorabi SM. Non-isothermal crystallization kinetics and fragility of Zr56Co28Al16 and Zr56Co22Cu6Al16 bulk metallic glasses. J Therm Anal Calorim. 2018;134:903–14.
Jiang SS, Zhu L, Zheng H, Wang YG. Kinetics of non-isothermal crystallization in FeNiPC(Nb) alloys. Thermochim Acta. 2020;684:178481.
Svoboda R, Malek J. Particle size influence on crystallization behavior of Ge2Sb2Se5 glass. J Non-Cryst Solids. 2012;358:276–84.
Svoboda R, Malek J. Crystallization kinetics of amorphous Se, Part 1. Interpretation of kinetic functions. J Therm Anal Calorim. 2013;114:473–82.
Svoboda R, Malek J. Crystallization kinetics of a-Se Part 2. Deconvolution of a complex process: the final answer. J Therm Anal Calorim. 2014;115:81–91.
Svoboda R, Malek J. Non-isothermal crystallization kinetics of As2Se3 glass studied by DSC. Thermochim Acta. 2014;579:56–63.
Svoboda R, Malek J. Particle size dependent isothermal crystallization kinetics in a Se-Te glassy system. Thermochim Acta. 2015;610:47–56.
Svoboda R, Malek J. Thermal behavior of Se-rich Ge2Sb2Se(5-y)Tey chalcogenide system. J Alloys Compd. 2015;627:287–98.
Svoboda R, Malek J. How nucleation-growth kinetics is influenced by initial degree of material crystallinity. Thermochim Acta. 2016;631:28–35.
Svoboda R, Malek J. Thermal behavior of Se-rich GeSb2Se(4-y)Tey (glassy) system. J Alloys Compd. 2016;670:222–8.
Brandova D, Svoboda R, Malek J. Influence of particle size on crystallization and relaxation behavior of Ge20Se4Te76 glass for infrared optics. J Non-Cryst Solids. 2016;433:75–81.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Joraid, A.A., Al-Marweny, A.A. & Al‑Maghrabi, M.A. Particle size effects on the crystallization kinetics of chalcogenide Se85Te10Sb5 glass. J Therm Anal Calorim 147, 3633–3645 (2022). https://doi.org/10.1007/s10973-021-10790-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-021-10790-7