Abstract
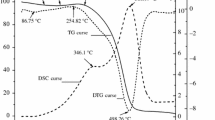
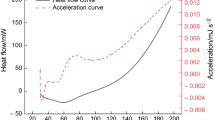
The thermokinetics and gaseous products of Jurassic coals collected from northern Shaanxi, China at four different heating rates were determined via thermogravimetry-Fourier transform infrared spectroscopy experiments. The results showed that the trends of thermogravimetric pyrolysis and oxidation curves were divided into three stages and five stages, respectively, and presented the same variations. As the heating rate increased, their curves moved the high temperature. In addition, the characteristic temperatures indicated that the coal samples had a relatively significant spontaneous combustion tendency. In the stage of water evaporation and gas desorption, the rates of formation of CO and CO2 went up promptly, and gaseous water produced during the oxidation reaction began to increase, but its generation rate was lower than that in the stage of oxygen absorption and mass increase. The apparent activation energy (Ea) showed a characteristic jump with increasing temperature, and the optimal mechanism function was chosen by integration and differentiation. Moreover, the relationship between Ea and lnA was linear, which indicated that there was a kinetic compensation effect.









Similar content being viewed by others
References
Deng J, Bai ZJ, Xiao Y, Shu CM, Bin LW. Effects of imidazole ionic liquid on macroparameters and microstructure of bituminous coal during low-temperature oxidation. Fuel. 2019;246:160–8.
Parada S, Czerski G, Dziok T, Grzywacz P, Makowska D. Kinetics of steam gasification of bituminous coals in terms of their use for underground coal gasification. Fuel Process Technol. 2015;130:282–91.
Xiang FP, He Y, Kumar S, Wang ZH, Liu LL, Huang ZY, et al. Influence of hydrothermal dewatering on trace element transfer in Yimin coal. Appl Therm Eng. 2017;117:675–81.
Bai ZJ, Wang CP, Deng J, Kang FR, Shu CM. Experimental investigation on using ionic liquid to control spontaneous combustion of lignite. Process Saf Environ Prot. 2020. https://doi.org/10.1016/j.psep.2020.06.017.
Qin SJ, Lu QF, Li YH, Wang JX, Zhao QJ, Gao K. Relationships between trace elements and organic matter in coals. J Geochem Explor. 2018;188:101–10.
Wang WS, Zhang CY. Evaluation of relative technological innovation capability: model and case study for China’s coal mine. Resour Policy. 2018;58:144–9.
Zhang ZW, Lu BW, Zhang LQ, Li XS, Luo C, Xu YQ, et al. Computational study on the effect of gasification reaction on pulverized coal MILD combustion diluted by N2 and CO2. Appl Therm Eng. 2019;158:113806.
Fu C, Anantharaman R, Jordal K, Gundersen T. Thermal efficiency of coal-fired power plants: from theoretical to practical assessments. Energy Convers Manage. 2015;105:530–44.
Vasil’ev AA, Pinaev AV, Trubitsyn AA, Grachev AY, Trotsyuk AV, Trilis AV. What is burning in coal mines: methane or coal dust? Combust Explo Shock Waves. 2017;53:8–14.
Deng J, Lei CK, Xiao Y, Cao K, Ma L, Wang WF, et al. Determination and prediction on “three zones” of coal spontaneous combustion in a gob of fully mechanized caving face. Fuel. 2018;211:458–70.
Wang K, Deng J, Zhang YN, Wang CP. Kinetics and mechanisms of coal oxidation mass gain phenomenon by TG-FTIR and in situ IR analysis. J Therm Anal Calorim. 2018;132:591–8.
Deng J, Xiao Y, Li QW, Lu JH, Wen H. Experimental studies of spontaneous combustion and anaerobic cooling of coal. Fuel. 2015;157:261–9.
Wang CJ, Yang SQ, Yang DD, Li XW, Jiang CL. Experimental analysis of the intensity and evolution of coal and gas outbursts. Fuel. 2018;226:252–62.
Yang JY, Wang GH, Zhang WG. The trace elements are bounded by organic functional groups in coal: a studying result based on FTIR analysis. Acta Geol Sin (Engl Ed). 2016;90:154–65.
Song HJ, Liu GR, Zhang JZ, Wu JH. Pyrolysis characteristics and kinetics of low rank coals by TG-FTIR method. Fuel Process Technol. 2017;156:454–60.
Morga R. Chemical structure of semifusinite and fusinite of steam and coking coal from the Upper Silesian Coal Basin (Poland) and its changes during heating as inferred from micro-FTIR analysis. Int J Coal Geol. 2010;84:1–15.
Deng J, Zhao JY, Huang AC, Zhang YN, Wang CP, Shu CM. Thermal behavior and microcharacterization analysis of second-oxidized coal. J Therm Anal Calorim. 2017;127:439–48.
Liu LL, Yuan Y, Sunel K, Wang ZH, He Y, Lv Y, et al. Catalytic effect of metal chlorides on coal pyrolsis and gasification part II. Effects of acid washing on coal characteristics. Thermochim Acta. 2018;666:41–50.
Deng J, Li B, Xiao Y, Ma L, Wang CP, Bin LW, et al. Combustion properties of coal gangue using thermogravimetry-Fourier transform infrared spectroscopy. Appl Therm Eng. 2017;116:244–52.
Deng J, Wang K, Zhang YN, Yang H. Study on the kinetics and reactivity at the ignition temperature of Jurassic coal in North Shaanxi. J Therm Anal Calorim. 2014;118:417–23.
Ma GX, Xia WC, Xie GY. Effect of particle shape on the flotation kinetics of fine coking coal. J Cleaner Prod. 2018;195:470–5.
Liu JX, Jiang YZ, Yao W, Jiang X, Jiang XM. Molecular characterization of Henan anthracite coal. Energy Fuels. 2019;33:6215–25.
Tian B, Qiao YY, Tian YY, Liu Q. Investigation on the effect of particle size and heating rate on pyrolysis characteristics of a bituminous coal by TG-FTIR. J Anal Appl Pyrolysis. 2016;121:376–86.
Kandasamy J, Iskender G, Stéphane B. High ash coal pyrolysis at different heating rates to analyze its char structure, kinetics and evolved species. J Anal Appl Pyrolysis. 2015;113:426–33.
Bai ZJ, Wang CP, Deng J. Analysis of thermodynamic characteristics of imidazolium‑based ionic liquid on coal. J Therm Anal Calorim. 2020;140:1957–65.
Deng J, Li QW, Xiao Y, Shu CM. Experimental study on the thermal properties of coal during pyrolysis, oxidation, and re-oxidation. Appl Therm Eng. 2017;110:1137–52.
Wang CP, Bai ZJ, Xiao Y, Deng J, Shu CM. Effects of FeS2 on the process of coal spontaneous combustion at low temperatures. Process Saf Environ Prot. 2020;142:165–73.
Ren SJ, Wang CP, Xiao Y, Deng J, Tian Y, Song JJ, et al. Thermal properties of coal during low temperature oxidation using a grey correlation method. Fuel. 2020;260:116287.
Wang CP, Hou YN, Xiao Y, Deng J, Shu CM, Xie XJ. Intrinsic characteristics combined with gaseous products and active groups of coal under low temperature oxidation. Combust. Sci. Technol. 2020:1753715.
Deng J, Bai ZJ, Xiao Y, Shu CM. Effects on the activities of coal microstructure and oxidation treated by imidazolium-based ionic liquids. J Therm Anal Calorim. 2018;133:453–63.
Yao HF, He BS, Ding GC, Tong WX, Kuang YC. Thermogravimetric analyses of oxy-fuel co-combustion of semi-coke and bituminous coal. Appl Therm Eng. 2019;156:708–21.
Huang JL, Liu JY, Chen JC, Xie WM, Kuo JH, Lu XW, et al. Combustion behaviors of spent mushroom substrate using TG-MS and TG-FTIR: thermal conversion, kinetic, thermokinetic and emission analyses. Bioresour Technol. 2018;266:389–97.
Deng J, Bai ZJ, Yang X, Laiwang B, Shu CM, Wang CP. Thermogravimetric analysis of the effects of four ionic liquids on the combustion characteristics and kinetics of weak caking coal. J Mol Liq. 2019;277:876–85.
Xu S, Liu JF, Cao W, Li YY, Cao WG. Experimental study on the minimum ignition temperature and combustion kinetics of coal dust/air mixtures. Powder Technol. 2017;317:154–61.
Yan FZ, Lin BQ, Xu J, Wang YN, Zhang XL, Peng SJ. Structural evolution characteristics of middle-high rank coal samples subjected to high-voltage electrical pulse. Energy Fuels. 2018;32:3263–71.
Adánez-Rubio I, Gayán P, Abad A, de Diego LF, García-Labiano F, Adánez J, et al. Kinetic analysis of a cu-based oxygen carrier: relevance of temperature and oxygen partial pressure on reduction and oxidation reactions rates in chemical looping with oxygen uncoupling (CLOU). Chem Eng J. 2014;256:69–84.
Liu X, Chen MQ, Wei YH. Kinetics based on two-stage scheme for co-combustion of herbaceous biomass and bituminous coal. Fuel. 2015;143:577–85.
Tsai YT, Yang Y, Wang CP, Shu CM, Deng J. Comparison of the inhibition mechanisms of five types of inhibitors on spontaneous coal combustion. Int J Energy Res. 2017;42:1158–71.
Wang HY, Chen C, Huang T, Gao W. CO2 emission of coal spontaneous combustion and its relation with coal microstructure, China. J Environ Biol. 2015;36:1017–24.
Bai ZJ, Wang CP, Deng J, Kang FR, Shu CM. Effects of ionic liquids on the chemical structure and exothermic properties of lignite. J Mol Liq. 2020. https://doi.org/10.1016/j.molliq.2020.113019.
Li QW, Xiao Y, Wang CP, Deng J, Shu CM. Thermokinetic characteristics of coal spontaneous combustion based on thermogravimetric analysis. Fuel. 2019;250:235–44.
Acknowledgements
This work was sponsored by National Key R&D Program of China (Grant No. 2018YFC0807900), National Natural Science Foundation of China (No. 51974234), and Shaanxi Province Innovative Talent Promotion Plan-Youth Science, Technology New Star Project (No. 2019KJXX-050).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, C., Hou, Y., Bai, Z. et al. Exploring thermokinetic behaviour of Jurassic coal during pyrolysis and oxidation. J Therm Anal Calorim 147, 1439–1453 (2022). https://doi.org/10.1007/s10973-020-10429-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-10429-z