Abstract
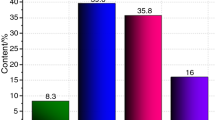
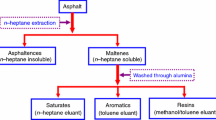
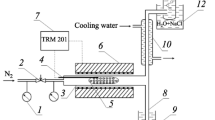
To discuss the thermal decomposition mechanism of asphalt materials, the kinetic parameters were calculated and their distribution models were established based on differential scanning calorimetry (DSC) test results of saturates, aromatics, resins and asphaltenes (SARA) fractions in asphalt. Results indicate that the thermal decomposition processes of SARA fractions are accompanied by complicated endothermic and exothermic reactions, and the exothermic reactions are predominant. The DSC curve is shifted to higher temperature when the heating rate is elevated, and the thermal effects, reaction progress and decomposition rate are increased simultaneously. Also, the Friedman–Reich–Levi (FRL) isoconversional method is suitable to calculate the kinetic parameters during the thermal decomposition of SARA fractions. The conversion ratio (α) of each SARA fraction shows obvious effects on the calculated activation energy (E). As the α of each SARA fraction is increased, the calculated E of saturates is more accurate at a lower α at the decomposition stages 1 and 3, but is always accurate at stage 2. Additionally, the calculated E is more accurate at the lower and higher α than that at the middle α at the two decomposition stages of aromatics. The calculated E of resins is fluctuated between 99 and 112 kJ mol−1, and its accuracy is satisfactory. The calculated E of asphaltenes is changed between 8 and 80 kJ mol−1, and is more accurate at the lower and higher α than that at the middle α. Finally, the distributed activation energy models of each SARA fraction are established to provide an insight into thermal decomposition mechanism of asphalt materials.












Similar content being viewed by others
References
Li Q, Li K, Zhao K, Sun G, Luo S. Fuel oil corrosion resistance of asphalt mixtures. Constr Build Mater. 2019;220:10–20.
Varfolomeev MA, Rakipov IT, Isakov DR. Characterization and kinetics of siberian and Tatarstan regions crude oils using differential scanning calorimetry. Liq Fuel Technol. 2015;33(8):865–71.
Wu K, Zhu K, Kang C. An experimental investigation of flame retardant mechanism of hydrated lime in asphalt mastics. Mater Des. 2016;103:223–9.
Xu T, Huang X. Study on combustion mechanism of asphalt binder by using TG–FTIR technique. Fuel. 2010;89(9):2185–90.
Gu F, Ma W, West RC, Taylor AJ, Zhang YQ. Structural performance and sustainability assessment of cold central-plant and in-place recycled asphalt pavements: a case study. J Clean Prod. 2019;208:1513–23.
Huang YW, Chen MQ, Li Y. An innovative evaluation method for kinetic parameters in distributed activation energy model and its application in thermochemical process of solid fuels. Thermochim Acta. 2017;655:42–51.
Zhao S, Pu WF. Low-temperature oxidation and thermal kinetics analysis of light and medium crude oils. Liq Fuel Technol. 2018;36(7):540–6.
Kök MV, Gul KG. Combustion characteristics and kinetic analysis of Turkish crude oils and their SARA fractions by DSC. J Therm Anal Calorim. 2013;114(1):269–75.
Zhao J, Huang X, Xu T. Combustion mechanism of asphalt binder with TG–MS technique based on components separation. Constr Build Mater. 2015;80:125–31.
Varfolomeev MA, Galukhin A, Nurgaliev DK. Thermal decomposition of Tatarstan Ashal’cha heavy crude oil and its SARA fractions. Fuel. 2016;186:122–7.
Aquino CB, Silva JMR, Oliveira MHR, Coriolano ACF, Delgado RCOB, Fernandes VJ, Araujo AS. Thermogravimetry applied for catalytic degradation of atmospheric residue of petroleum on mesoporous catalyst. J Therm Anal Calorim. 2019;136:2139–44.
Singh J, Kumar S, Garg MO. Kinetic modelling of thermal cracking of petroleum residues: a critique. Fuel Process Technol. 2012;94:131–44.
Vyazovkin S, Chrissafis K, Lorenzo ML. ICTAC Kinetics Committee recommendations for collecting experimental thermal analysis data for kinetic computations. Thermochim Acta. 2014;590:1–23.
Kok MV, Varfolomeev MA, Nurgaliev DK. Low-temperature oxidation reactions of crude oils using TGA–DSC techniques. J Therm Anal Calorim. 2020;141:775–81.
Popescu C. Integral method to analyze the kinetics of heterogeneous reactions under non-isothermal conditions a variant on the Ozawa–Flynn–Wall method. Thermochim Acta. 1996;285(2):309–23.
Guo S, He J, Luo W. Research on the thermal decomposition reaction kinetics and mechanism of pyridinol-blocked isophorone diisocyanate. Materials. 2016;9(2):110.
Vyazovkin S. Modification of the integral isoconversional method to account for variation in the activation energy. J Comput Chem. 2001;22(2):178–83.
Cai J, Chen S. A new iterative linear integral isoconversional method for the determination of the activation energy varying with the conversion degree. J Comput Chem. 2009;30(13):1986–91.
Kasyap S, Patel AT, Pratap A. Crystallization kinetics of Ti20 Zr20 Cu60 metallic glass by isoconversional methods using modulated differential scanning calorimetry. J Therm Anal Calorim. 2014;116(3):1325–36.
Vyazovkin S, Sbirrazzuoli N. Isoconversional analysis of the nonisothermal crystallization of a polymer melt. Macromol Rapid Commun. 2015;23(13):766–70.
Flynn JH. The temperature integral-its use and abuse. Thermochim Acta. 1997;300(1–2):83–92.
Vyazovkin S, Wight CA. Model-free and model-fitting approaches to kinetic analysis of isothermal and nonisothermal data. Thermochim Acta. 1999;340–341:53–68.
Guo FQ, Dong YP, Lv ZC. Kinetic behavior of biomass under oxidative atmosphere using a micro-fluidized bed reactor. Energy Convers Manag. 2016;108:210–8.
Ma L, Wang D, Wang Y. Synchronous thermal analyses and kinetic studies on a caged-wrapping and sustained-release type of composite inhibitor retarding the spontaneous combustion of low-rank coal. Fuel Process Technol. 2017;157:65–75.
Meng FR, Zhou YJ, Liu JY. Thermal decomposition behaviors and kinetics of carrageenanpoly vinyl alcohol biocomposite film. Carbohydr Polym. 2018;201:96–104.
Gao J, Man X, Shen J. Synthesis of pyrophoric active ferrous sulfide with oxidation behavior under hypoxic conditions. Vacuum. 2017;143:386–94.
Mehmood MA, Ye G, Luo H. Pyrolysis and kinetic analyses of Camel grass (Cymbopogon schoenanthus) for bioenergy. Bioresour Technol. 2017;228:18–24.
Wu W, Mei Y, Zhang L. Effective activation energies of lignocellulosic biomass pyrolysis. Energy Fuel. 2014;28(6):3916–23.
Vyazovkin S, Burnham AK, Criado JM. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520(1–2):1–19.
Pavel H. Temperature dependence of activation energy of endothermic processes and related imperfections of non-isothermal kinetic evaluations. J Therm Anal Calorim. 2017;129(1):609–14.
José AC, Conesa JA. Mathematical considerations for nonisothermal kinetics in thermal decomposition. J Anal Appl Pyrol. 2005;73(1):85–100.
Carrier M, Auret L, Bridgwater A, Knoetze JH. Using apparent activation energy as a reactivity criterion for biomass pyrolysis. Energy Fuels. 2016;30:7834–41.
Wang S, Dai G, Yang H. Lignocellulosic biomass pyrolysis mechanism: a state-of-the-art review. Prog Energy Combust Sci. 2017;62:33–86.
Scott SA, Dennis JS, Davidson JF. An algorithm for determining the kinetics of devolatilisation of complex solid fuels from thermogravimetric experiments. Chem Eng Sci. 2006;61(8):2339–48.
Arenas CN, Navarro MV, Martínez JD. Pyrolysis kinetics of biomass wastes using isoconversional methods and the distributed activation energy model. Bioresour Technol. 2019;288:121485.
Ahmad MS, Mehmood MA, Liu CG. Bioenergy potential of Wolffia arrhiza appraised through pyrolysis, kinetics, thermodynamics parameters and TG-FTIR-MS study of the evolved gases. Bioresour Technol. 2018;253:297–303.
Wu K, Zhu K, Han J, Wang JC, Huang ZY, Liang P. Non-isothermal kinetics of styrene butadiene styrene asphalt combustion. Chin Phys. 2013;22(6):06881.
Huang JL, Liu JY, Chen JC. Combustion behaviors of spent mushroom substrate using TG-MS and TG-FTIR: thermal conversion, kinetic, thermo-dynamic and emission analyses. Bioresour Technol. 2018;266:389–97.
Cai J, Xu D, Dong ZJ. Processing thermogravimetric analysis data for isoconversional kinetic analysis of lignocellulosic biomass pyrolysis: case study of corn stalk. Renew Sustain Energy Rev. 2018;82(3):2705–15.
Yao H, Dai Q, You ZP. Molecular dynamics simulation of physicochemical properties of the asphalt model. Fuel. 2016;164:83–93.
Guida MY, Bouaik H, El Mouden L, Moubarik A, Aboulkas A. Utilization of starink approach and avrami theory to evaluate the kinetic parameters of the pyrolysis of olive mill solid waste and olive mill wastewater. J Adv Chem Eng. 2017;7:155.
Shi H, Xu T, Zhou P. Combustion properties of saturates, aromatics, resins, and asphaltenes in asphalt binder. Constr Build Mater. 2017;136:515–23.
Xu J, Jiang Y, Zhang T. Synthesis of UV-curing waterborne polyurethane-acrylate coating and its photopolymerization kinetics using FT-IR and photo-DSC methods. Prog Org Coat. 2018;122:10–8.
Cai J, Wu W, Liu R. An overview of distributed activation energy model and its application in the pyrolysis of lignocellulosic biomass. Renew Sustain Energy Rev. 2014;36:236–46.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 51978340), Doctorate Fellowship Foundation of Nanjing Forestry University (No. 2170600095) and Postgraduate Research and Practice Innovation Program of Jiangsu Province (No. KYCX18_0971), and a project funded by the National First-class Disciplines (PNFD). Also we would like to thank Advanced Analysis and Testing Center of Nanjing Forestry University for the assistance in experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xia, W., Wang, S., Wang, H. et al. Thermal effects of asphalt SARA fractions, kinetic parameter calculation using isoconversional method and distribution models. J Therm Anal Calorim 146, 1577–1592 (2021). https://doi.org/10.1007/s10973-020-10152-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-10152-9