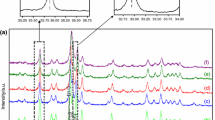
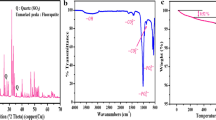
Abstract
The thermochemical and kinetics study of the attack of a synthetic fluorapatite (Fap) by a 10 mass% H2SO4 sulfuric acid solution was performed first at 25 °C using C-80 SETARAM microcalorimeter with reversal cells. The results are repetitive only for few amounts of Fap and the global enthalpy of the attack equals − 401.4 ± 9.7 kJ mol−1. The recorded curves and thermogenesis show one peak corresponding to the formation of anhydrous calcium sulfate (AH). The Avrami model has been used in order to determine the Avrami constants (k and n). The deconvoluted curves agree with a homogeneous kinetic scheme based on two successive reactions of order 1 with respect to calcium involving dissolution and precipitation phenomena. The precipitation enthalpy of AH deduced from iteration is close to the one determined experimentally and the sum of the reaction enthalpies does not differ from the global enthalpy determined by integrating the rough signal by more than 2.8%. Increasing temperature led to an increase in the attack rate, and kinetic results agree with the shrinking core model with a mixture of both diffusion through an ash layer and chemical reactions control. The two resulting apparent activation energies are 34.4 and 41.0 kJ mol−1, which are in the range determined by the isoconversional model [16.7–48.8 kJ mol−1].


















Similar content being viewed by others
References
Dorozhkin SV. A review on the dissolution models of calcium apatites. Prog Cryst Growth Charact Mater. 2002;44:45–61.
Harouiya N, Chairat C, Köhler SJ, Gout R, Oelhers EH. The dissolution kinetic and apparent solubility of natural apatite in closed reactors at temperatures from 5 to 50 °C and pH from 1 to 6. Chem Geol. 2007;244:554–68.
Gioia F, Mura G, Viola A. Analysis, simulation and optimization of hemihydrate process for the production of phosphoric acid from calcareous phosphites. Ind Eng Chem Process Des Dev. 1977;16(3):390–9.
Ashraf M, Zafar ZI, Ansari TM, Ahmed F. Selective leaching kinetics of calcareous phosphate rock in phosphoric acid. J Appl Sci. 2005;5:1722–7.
Soussi-Baatout A, Ibrahim K, Khattech I, Jemal M. Attack of Tunisian phosphate ore by phosphoric acid: kinetic study by means of differential reaction calorimetry. J Therm Anal Calorim. 2016;124:1671–8.
Sevim F, Sarac H, Kocakerim MM, Yartasi A. Dissolution kinetics of phosphate ore in H2SO4 solutions. Ind Eng Chem Res. 2003;42:2052–7.
Belgacem B, Leveneur S, Chlendi M, Estel L, Bagane M. The aid of calorimetry for kinetic and thermal study. J Therm Anal Calorim. 2019. https://doi.org/10.1007/s10973-018-7157-3.23456789.
Jemal M, Ben Cherifa A, Khattech I, Natahomvukiye I. Standard enthalpies of formation and mixing of hydroxy-, and fluorapatites. J Thermochim Acta. 1995;259:13–21.
Brahim K, Khattech I, Dubes JP, Jemal M. Etude cinétique et thermodynamique de la dissolution de la fluorapatites dans l’acide phosphorique à 25°C. Thermochim Acta. 2005;436:43–50.
Antar K, Brahim K, Jemal M. Etude cinétique et thermodynamique de l’attaque d’une fluorapatite par des mélanges d’acides sulfurique et phosphorique à 25°C. Thermochim Acta. 2006;449:35–41.
Heughebaert JC. Contribution à l’étude de l’évolution des orthophosphates de calcium précipités amorphes en orthophosphates apatitiques. Ph.D. thesis, Institut national polytechnique de Toulouse, Toulouse; 1977.
Prener JS. Nonstoichiometry in calcium chlorapatite. J Solid State Chem. 1971;3:49–55.
Sands DE. Weighting factors in least squares. J Chem Educ. 1974;51:473–4.
Pattengill MD, Sands DE. Statistical significance of linear last squares parameters. J Chem Educ. 1979;56:244–7.
Avrami M. Granulation, phase change, and microstructure kinetics of phase change III. J Chem Phys. 1941;9:177–84.
Liu M, Zhao Q, Wang Y, Zhang C, Mo Z, Cao S. Melting behaviors, isothermal and non-isothermal crystallization kinetics of nylon 1212. Polymer. 2003;44:2537–45.
Yavuz M, Maeda H, Vance L, Liu HK, Dou SX. Phase development and kinetics of high temperature Bi-2223 phase. J Alloys Compd. 1998;281:280–9.
Perlovich GL, Bauer-Brandl A. The melting process of acetylsalicylic acid single crystals. J Therm Anal Calorim. 2001;63:653–61.
Kabai J. Determination of specific activation energies of metal oxides and metal oxide hydrates by measurement of the rate of dissolution. Acta Chim Acad Sci Hung. 1973;78:57–73.
Vaimakis TC, Economou ED, Trapalis CC. Calorimetric study of dissolution kinetics of phosphorite in diluted acetic acid. J Therm Anal Calorim. 2008;92(3):783–9.
Fertani-Gmati M, Jemal M. Thermochemistry and kinetics of silica dissolution in NaOH aqueous solution. Thermochim Acta. 2001;513:43–8.
Okur H, Tekin T, Ozer AK, Bayramoglu M. Effect of ultrasound on the dissolution of colemanite in H2SO4. Hydrometallurgy. 2002;67:79–86.
Zendah H, Khattech I, Jemal M. Thermochemical and kinetic studies of the acid attack of “B” type carbonate fluorapatites at different temperatures (25–55)°C. Thermochim Acta. 2013;565:46–51.
Papon P, Leblond J, Meijer PHE. The physics of phase transitions concepts and applications. 2nd ed. Berlin: Springer; 2006.
Hubert S. Transition de phases solides induites par un procédé de compression directe: Application à la cféine et à la carbamazépine. Ph.D. thesis, University of Lyon; 2012.
Kuga N, Sêstak J. Thermoanalytical kinetics and physico-geometry of the nonisothermal crystallization of glasses. Bol Soc Esp Ceram Vidr. 1992;31:185–90.
Fosting ER. Phase transformation kinetics and microstructure of carbide and diboride based ceramics. Ph.D. thesis, Fakultät für Bergbau, Hüttenwesen und Maschinenwesen of the Technische Universität Clausthal; 2005.
Brahmia N. Contribution à la modélisation de la cristallisation des polymères sous cisaillement: application à l’injection des polymères semi-cristallins.Ph. D. thesis, Institut National des Sciences Appliquées de Lyon; 2007.
Rao CNR, Rao KJ. Phase transitions in solids, an approach to the study of the chemistry and physics of solids. New York: McGraw-Hill Inc; 1978.
Antar K, Jemal M. Kinetics and thermodynamics of the attack of fluorapatite by a mixture of sulfuric and phosphoric acids at 55°C. Thermochim Acta. 2007;452(1):71–5.
Mandal PK, Mandal TK. Anion water in gypsum (CaSO4·2H2O) and hemihydrate (CaSO4·1/2H2O). Cem Concr Res. 2002;32:313–6.
Zendah H, Contribution à l’étude thermochimique et cinétique de l’attaque par l’acide phosphorique de fluorapatites synthétiques variablement carbonatées, Ph.D. thesis, Université de Tunis El Manar, Tunis; 2013.
Brahim K. Contribution à l’étude thermodynamique et cinétique de l’attaque phosphorique d’une fluorapatite: Application à un phosphate naturel, Ph.D. thesis, Université de Tunis El Manar, Tunis; 2006.
Antar K, Jemal M. Kinetics and thermodynamics of the attack of a phosphate ore by acid solutions at different temperatures. Thermochim Acta. 2008;474:32–5.
Levenspiel O. Chemical reaction engineering. 3rd ed. New York: Wiley; 1999.
Sohn H, Wadsworth ME. Rate processes of extractive metallurgy. New York: Plenum Press; 1979.
Habashi F. Principles of extractive metallurgy. New York: Gordon and Breach; 1979.
Tekin G, Onganer Y, Alkan M. Dissolution kinetics of ulexite in ammonium chloride solution. Can Metall Q Can J Metall Mater Sci. 2013;37(2):91–7.
Gharabaghi M, Irannajad M, Noaparast M. A review of the beneficiation of calcareous phosphate ores using organic acid leaching. Hydrometallurgy. 2010;103(1–4):96–107.
Zafar ZI. Determination of semi empirical kinetic model for dissolution of bauxite ore with sulfuric acid: parametric cumulative effect on the Arrhenius parameters. J Chem Eng. 2008;141:233–41.
Souza AD, Pina PS, Leȃo VA, Silva CA, Siqueira PF. The leaching kinetics of a zinc sulphide concentrate in acid ferric sulphate. Hydrometallurgy. 2007;89:72–81.
Abdel-Aal EA, Rashed MM. Kinetic study on the leaching of spent nickel oxide catalyst with sulfuric acid. Hydrometallurgy. 2004;74:189–94.
Calmanovici CE, Gilot B, Laguérie C. Mechanism and kinetics for the dissolution of apatitic materials in acid solutions. Braz J Chem Eng. 1997;14(2):95–102.
Sbirrazzuoli N, Brunel D, Elegant L. Different kinetic equations analysis. J Therm Anal Calorim. 1992;38:1509–24.
Vyazovkin S. Evaluation of activation energy of thermally stimulated solid-state reactions under arbitrary variation of temperature. J Comput Chem. 1997;18:393–402.
Fertani-Gmati M, Jemal M. Thermochemical and kinetic investigations of amorphous silica dissolution in NaOH solutions. J Therm Anal Calorim. 2016;123:757–65.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aouadi-Selmi, H., Antar, K. & Khattech, I. Thermochemical and kinetic study of the attack of fluorapatite by sulfuric acid solution at different temperatures. J Therm Anal Calorim 141, 807–817 (2020). https://doi.org/10.1007/s10973-019-09044-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-09044-4