Abstract
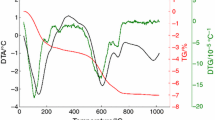
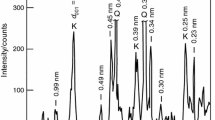
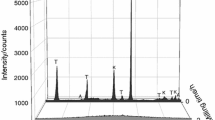
A non-isothermal kinetic analysis of the sintering process of an illitic clay is studied by thermodilatometry. For this study, illitic clay with over 80 mass% of illite content, originated in the Füzérradvány location in northeastern Hungary, is used as basic material. The measurements are performed using a push-rod dilatometer on compact samples with heating rates from 1 to 10 °C min−1 in dynamic N2 atmosphere. The Kissinger method is used for the parameterization of the process. The results show that the reaction sintering runs in several overlapping steps. The determined values of the apparent activation energy of the first step and second step are EA = (625 ± 18) kJ mol−1 and EA = (575 ± 14) kJ mol−1, respectively. The results also show that both reactions could be characterized by the thickening of long cylinders (needles) or growth of needles and plates of finite long dimensions.






Similar content being viewed by others
References
Rice RW. Ceramic fabrication technology. Boca Raton: CRC Press; 2002.
Kang S-JL. Sintering: densification, grain growth and microstructure. Oxford: Elsevier Butterworth-Heinemann; 2004.
Húlan T, Trník A, Medveď I. Kinetics of thermal expansion of illite-based ceramics in the dehydroxylation region during heating. J Therm Anal Calorim. 2017;127:1–8.
Bohor BF. High-temperature phase development in illitic clays. Clays Clay Miner. 1963;12:233–46.
Aras A. The change of phase composition in kaolinite- and illite-rich clay-based ceramic bodies. Appl Clay Sci. 2004;24:257–69.
Khalfaoui A, Kacim S, Hajjaji M. Sintering mechanism and ceramic phases of an illitic-chloritic raw clay. J Eur Ceram Soc. 2006;26:161–7.
Furlong RB. Electron diffraction and micrographic study of the high-temperature changes in illite and montmorillonite under continuous heating conditions. Clays Clay Miner. 1967;15:87–101.
Sedmale G, Sperberga I, Sedmalis U, Valancius Z. Formation of high-temperature crystalline phases in ceramic from illite clay and dolomite. J Eur Ceram Soc. 2006;26:3351–5.
Carroll DL, Kemp TF, Bastow TJ, Smith ME. Solid-state NMR characterisation of the thermal transformation of a Hungarian white illite. Solid State Nucl Magn Reson. 2005;28:31–43.
Wattanasiriwech D, Srijan K, Wattanasiriwech S. Vitrification of illitic clay from Malaysia. Appl Clay Sci. 2009;43:57–62.
Wang G, Wang H, Zhang N. In situ high temperature X-ray diffraction study of illite. Appl Clay Sci. 2017;146:254–63.
Ptáček P, Křečková M, Šoukal F, Opravil T, Havlica J, Brandštetr J. The kinetics and mechanism of kaolin powder sintering I. The dilatometric CRH study of sinter-crystallization of mullite and cristobalite. Powder Technol. 2012;232:24–30.
Emmerich W-D, Hayhurst J, Kaisersberger E. High temperature dilatometer study of special ceramics and their sintering kinetics. Thermochim Acta. 1986;106:71–8.
Knapek M, Húlan T, Minárik P, Dobroň P, Štubňa I, Stráská J, et al. Study of microcracking in illite-based ceramics during firing. J Eur Ceram Soc. 2016;36:221–6.
Húlan T, Trník A, Štubňa I, Bačík P, Kaljuvee T, Vozár L. Development of Young’s modulus of illitic clay during heating up to 1100 °C. Mater Sci Medzg. 2015;21:429–34.
Ptáček P, Šoukal F, Opravil T, Nosková M, Havlica J, Brandštetr J. The kinetics of Al-Si spinel phase crystallization from calcined kaolin. J Solid State Chem. 2010;183:2565–9.
Liu YF, Liu XQ, Tao SW, Meng GY, Sorensen OT. Kinetics of the reactive sintering of kaolinite-aluminum hydroxide extrudate. Ceram Int. 2002;28:479–86.
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520:1–19.
Karamanov A, Pelino M. Sinter-crystallization in the diopside-albite system. Part II. Kinetics of crystallization and sintering. J Eur Ceram Soc. 2006;26:2519–26.
Lopes AAS, Monteiro RCC, Soares RS, Lima MMRA, Fernandes MHV. Crystallization kinetics of a barium-zinc borosilicate glass by a non-isothermal method. J Alloys Compd. 2014;591:268–74.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Ondro T, Trník A. Kinetic behaviour of thermal transformations of kaolinite. In: AIP conference proceedings 2018;1988.
Ondro T, Trník A. Non-isothermal kinetic analysis of processes occurring during thermal treatment of kaolinite. In: AIP conference proceedings 2017;1866.
Augis JA, Bennett JE. Calculation of the Avrami parameters for heterogeneous solid state reactions using a modification of the Kissinger method. J Therm Anal. 1978;13:283–92.
Perejón A, Sánchez-Jiménez PE, Criado JM, Pérez-Maqueda LA. Kinetic analysis of complex solid-state reactions. A new deconvolution procedure. J Phys Chem B. 2011;115:1780–91.
Málek J. The applicability of Johnson-Mehl-Avrami model in the thermal analysis of the crystallization kinetics of glasses. Thermochim Acta. 1995;267:61–73.
Acknowledgements
This research was supported by the Czech Science Foundation, Grant No. GA17-16772S.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ondro, T., Húlan, T., Al-Shantir, O. et al. Kinetic analysis of the formation of high-temperature phases in an illite-based ceramic body using thermodilatometry. J Therm Anal Calorim 138, 2289–2294 (2019). https://doi.org/10.1007/s10973-019-08781-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08781-w