Abstract
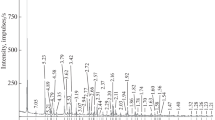
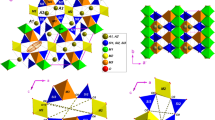
The synthetic samples of nickel olivine were measured in the temperature range 100–630 K by the X-ray powder diffraction method. Temperature dependencies of molar volumes and coefficients of bulk thermal expansion of liebenbergite were determined. Interpolation and extrapolation of the experimental data were performed by the procedure based on the Debye–Mie–Gruneisen theory of solid body in the range from 50 to 2000 K, and the Gruneisen coefficient and Debye temperature were calculated. Heat capacity and its behavior in accordance with temperature were evaluated.


Similar content being viewed by others
References
Akiyama K, Nagano I, Shida M, Ota S. Thermal barrier coating material. United States Patent. 2009; Patent No: US 7,622,411 B2.
Bass JD, Weidner DJ, Hamaya N, Ozima M, Akimoto S. Elasticity of the olivine and spinel polymorphs of Ni2SiO4. Phys Chem Miner. 1984;10(261):272.
Boström D. Single-crystal X-ray diffraction studies of synthetic Ni-Mg olivine solid solutions. Am Miner. 1987;72:965–72.
Brown G. The crystal chemistry of the olivines. Ph.D. thesis. Virginia Polytechnic Institute and State University, Blacksburg, Virginia; 1970.
Burns R. Site preferences of Ni2+ and Co2+ in clinopyroxenes roxenes and olivine: limitations of the statistical approach. Chem Geol. 1972;9:67–73.
Deer W, Howie R, Zussman J. An introduction to the rock-forming minerals. 2nd ed. Harlow: Longman; 1992. ISBN 0-582-30094-0.
Doroshev A, Kuznetsov G, Galkin V. The calculation of Gruneisen coefficient and characteristic Debye temperature from the data on thermal expansion and capacity. Zhurnal Fizicheskoi Khimii. 1988;LXII 3:823–5.
Frost DJ. The structure and sharpness of the (Mg, Fe)2SiO4 phase transformations in the transition zone. Earth Planet Sci Lett. 2003;216:313–28.
Galkin VM, Doroshev AM, Babich YV. Thermal expansion of coesite. Geokhimiya. 1987;N11:1645–6.
Galkin V, Gartvich Y. Thermal expansion and evaluation ion of almandine heat capacity. J Therm Anal Calorim. 2015;122(3):1239–44.
Galkin V, Kuznetsov G, Turkin A. Thermal expansion of ZnSiO3 high-pressure phases. Phys Chem Miner. 2007;34(6):377–81.
Hakli TA, Wright TL. The fractionation of nickel between olivine and augite as a geothermometer. Geochim Cosmochim Acta. 1967;31(5):877–84.
Henderson CMB, Redfern SAT, et al. Composition and temperature dependence of cation ordering in Ni–Mg olivine solid solutions: a time-of-flight neutron powder diffraction and EXAFS study. Am Mineral. 2001;86:1170–87.
Hirschman M. Thermodynamics of multicomponent olivines and the solution properties of (Ni, Mg, Fe)2SiO4 and (Ca, Mg, Fe)2SiO4 olivines. Am Miner. 1991;76:1232–48.
Koshlyakova NN, Zubkova NV, et al. Crystal chemistry of vanadate garnets from old metallurgical slags Lavrion, Greece. Neues Jahrbuch für Mineralogie - Abhandlungen: J Mineral Geochem. 2017;194:19–25.
Kuskov OL, Fabrichnaya OB. Phase diagrams of binary systems Mg2SiO4–Co2SiO4 and Fe2SiO4–Ni2SiO4 at P-T parameters of the upper mantle. Geochem J Russ Acad Sci. 1985;11:1551–66.
Lager GA, Meagher EP. High temperatures structural study of six olivines. Am Miner. 1978;63:365–77.
Lieberman RC. Elasticity of olivine (a), beta (/), spinel (y) polymorphisms of germanates and silicates. Geophys J R Astron Soc. 1975;42:899–929.
Ma CB. Phase equilibria and crystal chemistry in the system SiO2–NiO–NiAl2O4. Ph.D. thesis, Harvard University, Cambridge, Massachusetts; 1972.
Ma CB. New orthorhombic phases on the join NiAl2O4 (spinel analog)–Ni2SiO4 (olivine analog): stability and implications to mantle mineralogy. Contrib Miner Petrol. 1974;45(3):257–79.
Matsui Y, Syono Y. Unit cell dimensions of some synthetic olivine group solid solutions. Geochem J. 1968;2:51–9.
Matsukage K, Nishihara Y, Karato S. Seismological signature of chemical differentiation of earths upper mantle. J Geophys Res. 2005;110(B12305):1–18.
Miller ML, Ribbe PH. Methods for determination of composition and intracrystalline cation distribution in Fe–Mn Fe–Ni silicate olivines. Am Miner. 1985;70:723–8.
Navrotsky A. Ni2SiO4 enthalpy of the olivine-spinel transition by solution calorimetry at 713 °C. Earth Planet Sci Lett. 1973;19:471–5.
Okada Y, Tokumaru Y. Precise determination of lattice parameter and thermal expansion coefficient of silicon between 300 and 1500 K. J Appl Phys. 1984;56:314.
O’Neil HSC. Free energies of formation of NiO, CoO Ni2SiO4 and Co2SiO4. Am Miner. 1987;72:3–4.
Ozima M. Growth of nickel olivine single crystals by the flux method. J Cryst Growth. 1976;33(1):193–5.
Rajamani V, Brown GE, Prewitt CE. Cation ordering in Ni–Mg olivine. Am Miner. 1975;60:292–9.
Robie RA, Hemingway BS. Heat capacity and entropy of Ni2SiO4-olivine from 5 to 1000 K and heat capacity of Co2SiO4 from 360 to 1000 K. Am Miner. 1984;69:1096–101.
Ringwood AE. Melting relationships of Ni–Mg olivines and some geochemical implications. Geochim Cosmochim Acta. 1956;10:297–303.
Ringwood AE. Prediction and confirmation of olivine—spinel transformation in Ni2SiO4. Geochim Cosmochim Acta. 1962;26:457–69.
Tredoux M, Zaccarini F, Garuti G, Miller D. Phases in the Ni–Sb–As system which occur in the Bon Accord oxide body, Bar-berton greenstone belt, South Africa. Mineral Mag. 1982;80:187–98.
Vokurka K, Rieder M. Thermal expansion and excess volumes of synthetic olivines on the Mg2SiO4–Ni2SiO4 Join. Neue Jahrb Miner Monatshefte. 1987; 3:97–106.
Watanabe H. Thermochemical properties of synthetic high-pressure compounds relevant to the earth’s mantle. In: Akimoto S, Manghnani MH, editors. High-pressure research in geophysics. Tokyo: Center for Academic Publications; 1982. p. 441–64.
Xiaofei P, Lange R, Moore G. A comparison of olivine-melt thermometers based on DMg and DNi. The effects of melt composition, temperature, pressure with applications to MORBS and hydrous arc basalts. Am Miner. 2017;102:4.
Zahn A, Schreiter P. Lattice constants and site preference in the system Ni2SiO4–Co2SiO4. Cryst Res Technol. 1988;23(1):69–75.
Zhang Y, Sun Q, Geng J. Olivine thermal diffusivity influencing factors. J Therm Anal Calorim. 2018;132(1):7–16.
Acknowledgements
We thank Tolstyh O. for the help in measurements and Kuznetsov G. for the fruitful cooperation in calculations. This work was supported by the Russian Foundation for Basic Research (№ 0330-2016-0016).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gartvich, Y., Galkin, V. Ni olivine: thermal behavior of liebenbergite. J Therm Anal Calorim 136, 2333–2339 (2019). https://doi.org/10.1007/s10973-018-7859-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7859-6