Abstract
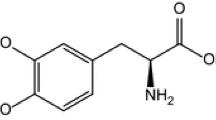
In this paper, we present the results obtained during the investigation of thermal stability of antiparkinsonian drug Levodopa in oxidative atmosphere and under non-isothermal conditions. As investigational tools, thermoanalytical methods were used along with ATR-FTIR spectroscopy and later completed with kinetic study for the main degradative process that occurs in the 260–330 °C temperature range, at four heating rates. Four kinetic methods were used as follows: ASTM E698 which leads to an activation energy of 209.72 kJ mol−1, while isoconversional methods suggested the values 177.6 ± 13.1 kJ mol−1 (Friedman) and 188.8 ± 4.5 kJ mol−1 (Kissinger–Akahira–Sunose), with a clear indication of multistep degradation. The last method used was NPK, which revealed that the degradation occurs in three steps, with different physical and chemical contributions with mean activation energy equal to 191.3 ± 9.5 kJ mol−1.







Similar content being viewed by others
Abbreviations
- A :
-
Pre-exponential factor (s−1)
- E :
-
Activation energy (kJ mol−1)
- R :
-
Universal gas constant (8.314 J mol−1 K−1)
- t :
-
Time (s)
- T :
-
Temperature (°C or K)
- f(α):
-
Differential form of kinetic mechanism function
- g(α):
-
Integral form of kinetic mechanism function
- α :
-
Conversion degree
- β :
-
Heating rate (°C min−1)
- k(T):
-
Rate constant
References
Shastry BS. Parkinson disease: etiology, pathogenesis and future of gene therapy. Neurosci Res. 2001;41(1):5–12.
Cannazza G, Di Stefano A, Mosciatti B, Braghiroli D, Baraldi M, Pinnen F, Sozio P, Benatti C, Parenti C. Detection of levodopa, dopamine and its metabolites in rat striatum dialysates following peripheral administration of L-DOPA prodrugs by mean of HPLC-EC. J Pharm Biomed Anal. 2005;36:1079–84.
Schapira AHV. Neurobiology and treatment of Parkinson’s disease. Trends Pharmacol Sci. 2009;30:41–7.
Safavi A, Tohidi M. Simultaneous kinetic determination of levodopa and carbidopa by H-point standard addition method. J Pharm Biomed Anal. 2007;44:313–8.
Lee KE, Choi YJ, Oh BR, Chun IK, Gwak HS. Formulation and in vitro/in vivo evaluation of levodopa transdermal delivery systems. Int J Pharm. 2013;456(2):432–6.
Li SF, Wu HL, Yu YJ, Li YN, Nie JF, Fu HY, Yu RQ. Quantitative analysis of levodopa, carbidopa and methyldopa in human plasma samples using HPLC-DAD combined with second-order calibration based on alternating trilinear decomposition algorithm. Talanta. 2010;81(3):805–12.
http://www.drugbank.ca/drugs/DB01235. Accessed 5 Nov 2016.
Wishart DS, Knox C, Guo AC, Shrivastava S, Hassanali M, Stothard P, Chang Z, Woolsey J. DrugBank: a comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res. 2006;34:D668–72.
Murata M. Pharmacokinetics of L-dopa. J Neurol. 2006;253:47–52.
Fuliaş A, Vlase G, Vlase T, Şuta L-M, Şoica C, Ledeţi I. Screening and characterization of cocrystal formation between carbamazepine and succinic acid. J Therm Anal Calorim. 2015;121(3):1081–6.
Ledeţi I, Vlase G, Ciucanu I, Olariu T, Fuliaş A, Şuta L-M, Belu I. Analysis of solid binary systems containing simvastatin. Rev Chim. 2015;66(2):240–3.
Fuliaş A, Soica C, Ledeţi I, Vlase T, Vlase G, Şuta L-M, Belu A. Characterization of pharmaceutical acetylsalicylic acid - theophylline cocrystal obtained by slurry method under microwave irradiation. Rev Chim. 2014;65(11):1281–4.
Ilici M, Bercean V, Venter M, Ledeti I, Olariu T, Suta L-M, Fulias A. Investigations on the thermal-induced degradation of transitional coordination complexes containing (3h-2-thioxo-1,3,4-thiadiazol-5-yl)thioacetate moiety. Rev Chim. 2014;65(10):1142–5.
Fuliaş A, Vlase G, Ledeţi I, Şuta L-M. Ketoprofen-cysteine equimolar salt: synthesis, thermal analysis, PXRD and FTIR spectroscopy investigation. J Therm Anal Calorim. 2015;121(3):1087–91.
Suta LM, Vlase G, Vlase T, Savoiu-Balint G, Olariu T, Belu I, Ledeti A, Murariu MS, Stelea L, Ledeti I. Thermal characterization of cholesterol in air vs. nitrogen atmosphere. Rev Chim. 2014;67(1):84–6.
Ledeti I, Simu G, Vlase G, Vlase T, Olariu T, Savoiu G, Suta L, Popoiu C, Fulias A. Ni (II) coordination compound with acetaminophen synthesis and characterization. Rev Chim. 2014;65(5):556–9.
Abdul Mujeeb VM, Muraleedharan K, Kannan MP, Ganga Devi T. The effect of particle size on the thermal decomposition kinetics of potassium bromate. J Therm Anal Calorim. 2011;108(3):1171–82.
Duce C, Vecchio Ciprioti S, Ghezzi L, Ierardi V, Tinè MR. Thermal behavior study of pristine and modified halloysite nanotubes: a modern kinetic study. J Therm Anal Calorim. 2015;121(3):1011–9.
Jiu HCJ, Sugahara T. Using the Friedman method to study the thermal degradation kinetics of photonically cured electrically conductive adhesives. J Therm Anal Calorim. 2015;119(1):425–33.
Shahcheraghi SH, Khayati GR, Ranjbar M. An advanced reaction model determination methodology in solid-state kinetics based on Arrhenius parameters variation: part II. Validation and application to crystallization of amorphous Cu4SO4O3. J Therm Anal Calorim. 2016;123(1):1–13.
Matos J, Oliveira JF, Magalhães D, Dubaj T, Cibulková Z, Šimon P. Kinetics of ambuphylline decomposition studied by the incremental isoconversional method. J Therm Anal Calorim. 2016;123(2):1031–6.
Šimon P, Dubaj T, Cibulková Z. Equivalence of the Arrhenius and non-Arrhenian temperature functions in the temperature range of measurement. J Therm Anal Calorim. 2015;120(1):231–8.
Suta L, Vlase G, Vlase T, Olariu T, Ledeti I, Belu I, Ivan C, Sarau CA, Savoiu-Balint G, Stelea L, Ledeti A. Solid state stability of cholesterol preliminary kinetic analysis. Rev Chim. 2016;67(1):113–5.
Budrugeac P. Phase transitions of a parchment manufactured from deer leather. J Therm Anal Calorim. 2015;120(1):103–12.
Brown ME, Maciejewski M, Vyazovkin S, Nomen R, Sempere J, Burnham A, Opfermann J, Strey R, Anderson HL, Kemmler A, Keuleers R, Janssens J, Desseyn HO, Li C-R, Tang TB, Roduit B, Malek J, Mitsuhashi T. Computational aspects of kinetic analysis part A: the ICTAC kinetics project-data, methods and results. Thermochim Acta. 2000;355:125–43.
Vyazovkin S, Burnham AK, Criado JM, Perez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520:1–19.
Dickinson CF, Heal GR. A review of the ICTAC kinetics project, 2000. Part 2. Non-isothermal results. Thermochim Acta. 2009;494(1–2):15–25.
Vyazovkin S. Computational aspects of kinetic analysis. Part C. The ICTAC kinetics project—the light at the end of the tunnel? Thermochim Acta. 2000;355(1–2):155–63.
Serra R, Sempere J, Nomen R. The non-parametric kinetics. A new method for the kinetic study of thermoanalytical data. J Therm Anal. 1998;52:933–43.
Serra R, Sempere J, Nomen R. A new method for the kinetic study of thermoanalytical data: the non-parametric kinetics model. Thermochim Acta. 1998;316:37–45.
Vlase T, Vlase G, Doca N, Bolcu C. Processing of non-isothermal TG data. Comparative kinetic analysis with NPK method. J Therm Anal Calorim. 2005;80:59–64.
Vlase T, Vlase G, Doca N, Ilia G, Fuliaş A. Coupled thermogravimetric-IR techniques and kinetic analysis by non-isothermal decomposition of Cd2+ and Co2+ vinyl-phosphonates. J Therm Anal Calorim. 2009;97:467–72.
Wall ME. Singular value decomposition and principal component analysis. In: Berrar DP, Dubitzky W, Granzow M, editors. A practical approach to microarray data analysis. Dordrecht: Kluwer-Norwel; 2003. p. 91–109.
Šesták J, Berggren G. Study of the kinetics of the mechanism of solid-state reactions at increasing temperatures. Thermochim Acta. 1971;3:1–12.
Ledeţi I, Vlase G, Vlase T, Fuliaş A. Kinetic analysis of solid-state degradation of pure pravastatin versus pharmaceutical formulation. J Therm Anal Calorim. 2015;121(3):1103–10.
Ledeţi I, Ledeţi A, Vlase G, Vlase T, Matusz P, Bercean V, Suta L-M, Piciu D. Thermal stability of synthetic thyroid hormone l-thyroxine and l-thyroxine sodium salt hydrate both pure and in pharmaceutical formulations. J Pharm Biomed Anal. 2016;125:33–40.
Friedman HL. New methods for evaluating kinetic parameters from thermal analysis data. J Polym Sci Polym Lett. 1969;7:41–6.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Akahira T, Sunose T. Research report, Trans joint convention of four electrical institutes. Chiba Inst Technol (Sci Technol). 1971;16:22–31.
O’Neil MJ, editor. The Merck Index—an encyclopedia of chemicals, drugs, and biologicals. 13th ed. Whitehouse Station: Merck and Co., Inc.; 2001. p. 979.
https://www.alfa.com/en/catalog/A11311/. Accessed 10 Dec 2016.
http://www.sigmaaldrich.com/catalog/product/usp/1361009?lang=en®ion=RO. Accessed 10 Dec 2016.
Ledeti A, Vlase G, Circioban D, Ledeti I, Dehelean C, Stelea L, Vlase T, Caunii A. Comparative stability of Levodopa under thermal stress in both oxidative and inert media. Rev Chim. 2016;69(12):2648–50.
Kura AU, Al Ali SHH, Hussein MZ, Fakurazi S, Arulselvan P. Development of a controlled-release anti-parkinsonian nanodelivery system using levodopa as the active agent. Int J Nanomed. 2013;8:1103–10.
Ozawa T. Kinetic analysis of derivative curves in thermal analysis. J Therm Anal. 1970;2(3):301–24.
Prime RB. Differential scanning calorimetry of the epoxy cure reaction. Polym Eng Sci. 1973;13(5):365–71.
Peyser P, Bascom WD. Kinetics of an anhydride-epoxy polymerization as determined by differential scanning calorimetry. In: Porter RS, Johnson JF editors. Analytical calorimetry. Vol. 3. Boston: Springer; 1974. p. 537–54.
Acknowledgements
This work was supported by the PN-II-RU-TE-2014-4-0515 to Adriana Ledeti, Gabriela Vlase, Denisa Circioban, and Ionut Ledeti.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ledeti, A., Olariu, T., Caunii, A. et al. Evaluation of thermal stability and kinetic of degradation for levodopa in non-isothermal conditions. J Therm Anal Calorim 131, 1881–1888 (2018). https://doi.org/10.1007/s10973-017-6671-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6671-z