Abstract
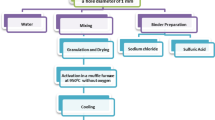
The scope of this investigation is to relate textural and surface characteristics of activated carbon prepared from Cerrejon coal (Colombia) to their methane quantity adsorption. Sub-bituminous coal from the Cerrejon coal mine in Colombia was used as a raw material for the preparation of pore size-controlled activated carbon adsorbents. A subsequent chemical treatment was carried out, after making a physical activation with CO2. Samples were treated with CO2 flow at 850 °C by varying activation time to achieve different burn-off activated carbons. The samples chemically activated with H3PO4 under CO2 atmosphere showed higher activation rates, surface area, and micropore volume compared to other activation methods, although not all the samples prepared by this method presented storage quantities methane in function of surface area development. Moreover, it was shown that using small proportion of KCl and H3PO4 creates an initial narrow microporosity. Further physical activation grantees better development of pore structure. In terms of pore size distribution, the combined preparation method resulted in a better and more homogenous pore size distribution than the conventional physical activation method. Controlling the pore size of activated carbon by this combined activation technique can be utilized for tuning the pore size distribution. Storage studies of methane up to 60 bar were realized. It was concluded that the high surface area and micropore volume of activated carbons do not unequivocally determine methane capacities. Adsorption microcalorimetry is a useful technique to follow the process of the adsorption reaction of CH4, and its results are in agreement with the results found study by adsorption at high pressure.





Similar content being viewed by others
References
Zheng Q, Zhu ZW, Wang X. Experimental studies of storage by adsorption of domestically used natural gas on activated carbon. Appl Therm Eng. 2015;77:134–41.
Jordi OS, Xavier G, Joan R. Environmental impacts of natural gas distribution networks within urban neighborhoods. Appl Energy. 2009;86:1915–24.
Wong JW. City-gas development in China-An N.G perspective. Energy Policy. 2010;38:2107–9.
Gao S. The research of adsorption model for methane/hydrogen and thermal effects on storage process. Jimei University of PRC, 2013 (Master’s thesis).
Biloé S, Goetz V, Guillot A. Optimal design of an activated carbon for an adsorbed natural gas storage system. Carbon. 2002;40:1295–308.
Chang KJ, Talu O. Behavior and performance of adsorption natural gas storage cylinders during discharge. Appl Therm Eng. 1996;16:359–74.
Rahman KA, Chakraborty A, Saha BB, Ng KC. On thermodynamics of methane+ carbonaceous materials adsorption. Heat Mass Transfer. 2012;55:565–73.
Rios RB, Bastos-Neto M, Amora MR Jr, Torres EB, Azevedo DCS, Calvacante C Jr. Experimental analysis of the efficiency on charge/discharge cycles in natural gas storage by adsorption. Fuel. 2011;90:113–9.
Policicchio A, MacCallini E, Agostino R, Ciuchi F, Aloise A, Giordano G. Higher methane storage at low pressure and room temperature in new easily scalable large-scale production activated carbon for static and vehicular applications. Fuel. 2013;104:813–21.
Quinn DF, Macdonald JA. Natural gas storage. Carbon. 1992;30:1097–103.
Rahman KA, Loh WS, Chakraborty A, Saha BB, Chun WG, Ng GC. Thermal enhancement of charge and discharge cycles for adsorbed natural gas storage. Appl Therm Eng. 2011;31:1630–9.
Ridha FN, Yunus RM, Rashid M, Ismail AF. Thermal transient behavior of an ANG storage during dynamic discharge phase at room temperature. Appl Therm Eng. 2007;37:55–62.
Sáez A, Toledo M. Thermal effect of the adsorption heat on an adsorbed natural gas storage and transportation. J Appl Therm Eng. 2009;39:2617–23.
Arami-Niya A, Wan Daud WMA, Farouq S. Comparative study of the textural characteristics of oil palm shell activated carbon produced by chemical and physical activation for methane adsorption. Chem Eng Res Des. 2011;89:657–64.
Marsh H, Rodríguez-Reinoso F. Activated carbon. Amsterdam: Elsevier; 2006.
Moreno-Castilla C, Carrasco-Marín F, López-Ramón MV, Alvarez-Merino MA. Chemical and physical activation of olive-mill waste water to produce activated carbons. Carbon. 2001;39:1415–20.
Afrane A. The evolution of the pore structure of coconut shells during the preparation of coconut shell-based activated carbons. Microporous Mesoporous Mater. 2008;112:284–90.
Allwar A, Noor AM, Nawi MABM. Textural characteristics of activated carbons prepared from oil palm shells activated with ZnCl2 and pyrolysis under nitrogen and carbon dioxide. J Phys Sci. 2008;19(2):93–104.
Gratuito MK, Panyathanmaporn T, Chumnanklang R, Sirinuntawittaya N, Dutta A. Production of activated carbon from coconut shell: optimization using response surface methodology. Bioresource Technol. 2008;99:4887–95.
Nakagawa Y, Molina-Sabio M, Rodríguez-Reinoso F. Modification of the porous structure along the preparation of activated carbon monoliths with H3PO4 and ZnCl2. Microporous Mesoporous Mater. 2007;103:29–34.
Prauchner M, Rodríguez-Reinoso F. Preparation of granular activated carbons for adsorption of natural gas. Microporous Mesoporous Mater. 2008;109:581–4.
Rouquerol J, Rouquerol F, Sing KS. Adsorption by powders and porous solids: principles, methodology and applications. London: Academic Press; 1999.
Şentorun-Shalaby Ç, Uçak-Astarlıogˇlu MG, Artok L, Sarici Ç. Preparation and characterization of activated carbons by one-step steam pyrolysis/activation from apricot stones. Microporous Mesoporous Mater. 2006;88:126–34.
Mozammel H, Masahiro O, Bhattacharya SC. Activated charcoal from coconut shell using ZnCl2 activation. Biomass Bioenergy. 2002;22:397–400.
Molina-Sabio M, Almansa C, Rodríguez-Reinoso F. Phosphoric acid activated carbon discs for methane adsorption. Carbon. 2003;41:2113–9.
Pillai R, Sunil P, Raksh J. Adsorption of carbon dioxide, methane, nitrogen, oxygen and argon in NaETS-4. Microporous Mesoporous Mater. 2008;113:268–76.
Hu Z, Guo H, Srinivasan MP, Yaming NA. Simple method for developing mesoporosity in activated carbon. Sep Purif Technol. 2003;31:47–52.
Ismadji S, Bhatia SK. A modified pore filling isotherm for liquid phase adsorption in activated carbon. Langmuir. 2003;17:1488–98.
Devarly PY, Kartika N, Indraswati S. Activated carbon from jackfruit peel waste by H3PO4 chemical activation: pore structure and surface chemistry characterization. Chem Eng J. 2008;140:32–42.
Suzuki RM, Andrade AD, Sousa JC, Rollemberg MC. Preparation and characterization of activated carbon from rice bran. Bioresource Technol. 2007;98:1985–91.
Thommes M, Kaneko K, Neimark AV, Olivier JP, Rodriguez-Reinoso F, Rouquerol J, Sing KSW. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl Chem. 2015;87(9–10):1051–69.
Yang T, Chong LA. Textural and chemical properties of zinc chloride activated carbons prepared from pistachio-nut shells. Mater Chem Phys. 2006;100:438–44.
Moreno-Piraján JC, García-Cuello VS, Giraldo L. Characterization of mordenite-supported Pd, Pt, and Ir determined by CO adsorption microcalorimetry and the dehydrogenation reaction of C3 alkanes. Top Catal. 2011;54:146–52.
Llewellyn P, Maurin G. Gas adsorption microcalorimetry and modelling to characterise zeolites and related materials. Chimie. 2005;8:283–302.
Spirewak BE, Dumesic JA. Applications of adsorption microcalorimetry for the characterization of metal-based catalyts. Themochimcia acta. 1998;312:95–104.
Acknowledgements
The authors thank the Universidad de los Andes (Bogotá, Colombia) and Universidad Nacional de Colombia (Sede Bogotá) for the framework agreement between the two institutions under which this research was developed. Special thanks go to the Faculty of Science and the Vice-Rectory of Research at the University of the Andes (Bogotá, Colombia) for funding this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moreno-Piraján, J.C., Bastidas-Barranco, M.J. & Giraldo, L. Preparation of activated carbons for storage of methane and its study by adsorption calorimetry. J Therm Anal Calorim 131, 259–271 (2018). https://doi.org/10.1007/s10973-017-6132-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6132-8