Abstract
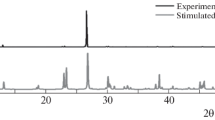
The sodium d-gluconate Na[d-C6H11O7](s) has been synthesized and characterized by elemental analysis and X-ray crystallography. X-ray single-crystal analysis reveals that the compound exhibits an obvious chelate property of d-gluconate anions to sodium cation, and the latter is coordinated to six d-gluconate anions. The lattice potential energy of the compound and ionic volume of the d-gluconate anion are obtained from crystallographic data. In accordance with law of Hess, a reasonable thermochemical cycle is designed and the standard molar enthalpy of formation of the compound Na[d-C6H11O7](s) is determined to be −(1472.68 ± 0.48) kJ mol−1 by use of an isoperibol solution-reaction calorimeter. Molar enthalpies of dissolution of Na[d-C6H11O7](s) at various molalities are measured at T = 298.15 K in the double-distilled water. According to Pitzer’s electrolyte solution theory, molar enthalpy of dissolution of the title compound at infinite dilution is determined to be (23.783 ± 0.128) kJ mol−1.





Similar content being viewed by others
References
Znad H, Markos J, Bales V. Production of gluconic acid from glucose by Aspergillus niger: growth and non-growth conditions. Process Biochem. 2004;39:1341–5.
Amin MA, Abd El Rehim SS, El-Lithy AS. Corrosion, passivation and breakdown of passivity of Al and Al–Cu alloys in gluconic acid solutions. Electrochim Acta. 2010;55:5996–6003.
Giroux S, Rubini P, Henry B, Aury S. Complexes of praseodymium(III) with d-gluconic acid. Polyhedron. 2000;19:1567–74.
Venkata Krishnan R, Jogeswararao G, Ananthasivan K. The standard molar enthalpies of formation of RE6UO12 (RE = La, Nd) by acid solution calorimetry. J Therm Anal Calorim. 2015;121:1375–82.
Gmati-Ben Khaled H, Khattech I, Jemal M. Standard molar enthalpy of formation of rubidium diphosphate. J Therm Anal Calorim. 2014;118:579–83.
Leinemann I, Timmo K, Grossberg M, Kaljuvee T, Tõnsuaadu K, Traksmaa R, Altosaar M, Meissner D. Reaction enthalpies of Cu2ZnSnSe4 synthesis in KI. J Therm Anal Calorim. 2015;119:1555–64.
Sun WJ, Liu F, Zhao WJ, Yang XW. Synthesis, characterization, and calorimetric investigation of ternary lanthanide complexes with benzoic acid and 2-phenylimidazo [4,5-f] 1,10-phenanthroline. J Therm Anal Calorim. 2015;119:671–80.
Li CH, Jiang JH, Yang P, Tao LM, Li X, Xiao SX, Peng X, Tao X, Xie JQ, Zhu Y, Xie MA, Li QG. Preparation, structure, and thermochemical properties of a copper(II) Schiff-base complex. J Therm Anal Calorim. 2015;119:1285–92.
Li B, Zhang YH, Kong YX, Di YY, Dou JM. Crystal structure and thermochemistry of a novel coordination compound 2-pyrazine carboxylate sodium. J Therm Anal Calorim. 2015;120:1027–34.
Kong YX, Di YY, Qi YD, Yang WW, Tan ZC. Low temperature heat capacities and standard molarenthalpy of formation of sodium benzoate C6H5COONa(s). Thermochim Acta. 2009;488:27–32.
Yang WW, Di YY, Kong YX, Guo XY, Tan ZC. Synthesis, characterization, and thermodynamic study of ammonium benzoate C7H5O2NH4(s). Thermochim Acta. 2010;502:14–9.
Littleton CD. A structure determination of the gluconate ion. Acta Cryst. 1953;6:775–81.
Lis T. Structure of sodium d-gluconate, Na[C6H11O7]. Acta Cryst. 1984;C40:376–8.
Lis T. Structure of lead(II) d-gluconate, Pb[C6H11O7]2. Acta Cryst. 1984;C40:374–6.
Jenkins HDB, Tudela D, Glasser L. Lattice potential energy estimation for complex ionic salts from density measurements. Inorg Chem. 2002;41(9):2364–7.
Glasser L, Jenkins HDB. Internally consistent ion volumes and their application in volume-based thermodynamics. Inorg Chem. 2008;47(14):6195–202.
Yao YB, Xie T, Gao YM. Handbook of physical chemistry. ShangHai: Shanghai Science and Technique Publishing House; 1985.
Chase MW. NIST-JANAF themochemical tables, fourth edition. J Phys Chem Ref Data Monogr. 1998;9:1–1951.
Pitzer KS, editor. Chapter 3: Ion interaction approach: theory and data correlation. In: Activity coefficients in electrolyte solutions, 2nd ed. Boca Raton: CRC Press; 1991. p. 75–153.
Silvester LF, Pitzer KS. Thermodynamics of electrolytes. 8. High-temperature properties, including enthalpy and heat capacity, with application to sodium chloride. J Phys Chem. 1977;81(19):1822–8.
Acknowledgements
This work is financially supported by the National Natural Science Foundations of China under the contract NSFC Nos. 21273171 and 21273100.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Di, YY., Zhang, YH., Liu, YP. et al. Crystal structure and thermodynamic properties of sodium d-gluconate Na[d-C6H11O7](s). J Therm Anal Calorim 127, 1835–1843 (2017). https://doi.org/10.1007/s10973-016-6033-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-6033-2