Abstract
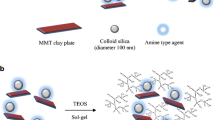
New zirconium-porous clay heterostructures (PCHs) were prepared using zirconium-tetramer-intercalated clay mineral as precursors and a subsequent reaction with alkylamine molecules and a silica source, such as tetraethyl orthosilicate. The organic molecules were removed by calcinations at temperatures above 550 °C. The precursors and resulting materials were systematically characterized using different techniques: XRD, XRF, 29Si MAS NMR, N2 adsorption, and TG-DTG. The thermal stability of the zirconium precursor and porous clay heterostructure was reported for the first time using in situ XRD high temperature. The zirconium content in the PCHs was tuned using the starting precursors with different zirconia percentages, and its presence improved the thermal stability, microtextural properties, and acidity of the PCH materials compared to the conventional PCH materials. The length of the alkyl amine chains used also affected the previously mentioned properties. A higher surface area of 950 m2 g−1 and pore volume of 0.801 cc g−1 were obtained using dodecylamine molecules and Zr-intercalated clay with a starting ratio of Zr (mole) to grams of clay of 6. The zirconium-porous clay heterostructures were stable up to 650 °C, with a total acidity concentration of 0.993 mol g−1 of PCH, in addition to strong Brönsted and Lewis acid sites, which were detected at 500 °C in vacuum.












Similar content being viewed by others
References
Vallet-Regi M, Balas F, Arcos D. Mesoporous materials for drug delivery. Angew Chem Int Ed. 2007;46:7548–56.
Bhattacharyya S, Lelong G, Saboungi ML. Recent progress in the synthesis and selected applications of MCM-41: a short review. J Exp Nanosci. 2006;1:375–95.
Raman NK, Anderson MT, Brinker CT. Template-based approaches to the preparation of nanoporous silicas. Chem Mater. 1996;8:1682–701.
Galarneau A, Barodawalla A, Pinnavaia TJ. Porous clay heterostructures formed by gallery-templated synthesis. Nature. 1994;374:529–31.
Pires J, Pinto M, Estella J, Echeverría JC. Characterization of the hydrophobicity of mesoporous silicas and clays with silica pillars by water adsorption and DRIFT. J Colloid Interface Sci. 2008;317:206–13.
Chmielarz L, Kuśtrowski P, Drozdek M, Dziembaj R, Cool P, Vansant EF. Selective catalytic oxidation of ammonia into nitrogen over PCH modified with copper and iron species. Catal Today. 2006;114:319–25.
Arellano-Cárdenas S, Gallardo-Velázquez T, Osorio-Revilla G, López-Cortez M. Preparation of a porous clay heterostructure and study of its adsorption capacity of phenol and chlorinated phenols from aqueous solutions. Water Environ Res. 2008;80:60–7.
Srithammaraj K, Magaraphan R, Manuspiya H. Modified porous clay heterostructures by organic–inorganic hybrids for nanocomposite ethylene scavenging/sensor packaging film. Packag Technol Sci. 2012;25:63–72.
Gârea SA, Mihai AI, Ghebaur A, Nistor C, Sârbu A. Porous clay heterostructures: a new inorganic host for 5-fluorouracil encapsulation. Int J Pharm. 2015;491:299–309.
Kooli F, Hian PC, Weirong Q, Alshahateet SF, Chen F. Effect of the acid activated clays on the properties of porous clay heterostructures. J Porous Mater. 2006;13:319–24.
Chmielarz L, Piwowarska Z, Kuśtrowski P, Gil B, Adamski A, Dudek B, Michalik M. Porous clay heterostructures (PCHs) intercalated with silica-titania pillars and modified with transition metals as catalysts for the DeNOx process. Appl Catal B Environ. 2009;91:449–59.
Polverejan M, Liu Y, Pinnavaia TJ. Aluminated derivatives of porous clay heterostructures (PCH) assembled from synthetic saponite clay: properties as supermicroporous to small mesoporous acid catalysts. Chem Mater. 2002;14:2283–8.
Pinto ML, Saini VK, Guil JM, Pires J. Introduction of aluminum to porous clay heterostructures to modify the adsorption properties. Appl Clay Sci. 2014;101:497–502.
Chmielarz L, Gil B, Kustrowski P, Piwowarska Z, Dudek B, Michalik M. Montmorillonite-based porous clay heterostructures (PCHs) intercalated with silica–titania pillars—synthesis and characterization. J Solid State Chem. 2009;182:1094–104.
Cecilia JA, García-Sancho C, Franco F. Montmorillonite based porous clay heterostructures: influence of Zr in the structure and acidic properties. Microporous Mesoporous Mater. 2013;176:95–102.
Yamaguchi T. Application of ZrO2 as a catalyst and a catalyst support. Catal Today. 1994;20:199–217.
Ohtsuka K. Preparation and properties of two-dimensional microporous pillared interlayered solids. Chem Mater. 1997;9:2039–50.
Kooli F, Jones W. Systematic comparison of a saponite clay pillared with Al and Zr metal oxides. Chem Mater. 1997;9:2913–20.
Pinto ML, Marques J, Pires J. Porous clay heterostructures with zirconium for the separation of hydrocarbon mixtures. Sep Purif Technol. 2012;98:337–43.
Kooli F. Porous clay heterostructures (PCHs) from Al13-intercalated and Al13-pillared montmorillonites: properties and heptane hydro-isomerization catalytic activity. Microporous Mesoporous Mater. 2014;184:184–92.
Kooli F, Yan L, Hbaieb K, Al-Faze R. Characterization and catalytic properties of porous clay heterostructures from zirconium intercalated clay and its pillared derivatives. Microporous Mesoporous Mater. 2016;226:482–92.
Kooli F, Sim TH, Jian D, Yan L, Alshahateet SF, Martin C, Rivers V. Zirconium nitrate solution as pillaring agent of montmorillonites clays. Clay Sci. 2006;12(2):301–5.
Awate SV, Waghmode SB, Patil KR, Agashe MS, Joshi PN. Influence of preparation parameters on characterization of zirconia-pillared clay using ultrasonic technique and its catalytic performance in phenol hydroxylation reaction. Korean J Chem Eng. 2001;18:257–62.
Toranzo R, Vicente MV, Banares-Munoz MA, Gandı LM, Gil A. Pillaring of saponite with zirconium oligomers. Microporous Mesoporous Mater. 1998;173:173–88.
Kooli F, Jones W. Al and Zr pillared acid-activated saponite clays: characterization and properties. J Mater Chem. 1998;8:2119–24.
Farfan EM, Sham E, Grange P. Pillared clay: preparation and characterization of zirconium pillared montmorillonite. Catal Today. 1992;15:515–26.
Cool P, Vansant EF. Preparation and characterization of zirconium pillared laponite and hectorite. Microporous Mater. 1996;6:27–36.
Bahranowski K, Włodarczyk W, Wisła-Walsh E, Gaweł A, Matusik J, Klimek A, Gil B, Michalik-Zym A, Dula R, Socha RP, Serwicka EM. [Ti, Zr]-pillared montmorillonite—a new quality with respect to Ti- and Zr-pillared clays. Microporous Mesoporous Mater. 2015;202:155–64.
Ursu AV, Jinescu G, Gros F, Nistor ID, Miron ND, Lisa G, Silion M, Djelveh G, Azzouz A. Thermal and chemical stability of Romanian bentonite. J Therm Anal Calorim. 2011;106:965–71.
Sun Kou MR, Mendioroz S, Guijarro MI. A thermal study of Zr-pillared montmorillonite. Themochim Acta. 1998;323:145–57.
Kooli F, Liu Y, Tan SX, Zheng J. Organoclays from alkaline-treated acid-activated clays. J Therm Anal Calorim. 2014;115:1465–75.
Zhu J, Shen W, Ma Y, Zhou Q, Yuan P, Liu D, He H. The influence of alkyl chain length on surfactant distribution within organo-montmorillonites and their thermal stability. J. Thermal Anal Calorim. 2016;109:301–9.
He H, Duchet J, Galy J, Gerard JF. Grafting of swelling clay materials with 3-aminopropyltriethoxysilane. J Colloid Interface Sci. 2005;288:171–6.
Belver C, Aranda P, Martin-Luengo MA, Ruiz-Hitzky E. New silica/alumina–clay heterostructures: properties as acid catalysts. Microporous Mesoporous Mater. 2012;147:157–66.
Wang F, Nimmo SL, Cao B, Mao C. Oxide formation on biological nanostructures via a structure-directing agent: towards an understanding of precise transcription. Chem Sci. 2012;3:2639–45.
Zapata PA, Belverb C, Quijada R, Aranda P, Ruiz-Hitzky E. Silica/clay organo-heterostructures to promote polyethylene–clay nanocomposites by in situ polymerization. Appl Catal A Gen. 2013;453:142–50.
Zimowska M, Pálková H, Madejová J, Dula R, Pamin K, Olejniczak Z, Gil B, Serwicka EM. Laponite-derived porous clay heterostructures: III. The effect of alumination. Microporous Mesoporous Mater. 2013;175:67–75.
Kooli F. Pillared montmorillonites from unusual antiperspirant aqueous solutions: characterization and catalytic tests. Microporous Mesoporous Mater. 2013;167:228–36.
Benjelloun M, Cool P, Linssen T, Vansant EF. Acidic porous clay heterostructures: study of their cation exchange capacity. Microporous Mesoporous Mater. 2001;49:83–94.
Pichowicz M. Mokaya. R. Porous clay heterostructures with enhanced acidity obtained from acid-activated clays. Chem Commun. 2001;20:2100–1.
Wang Y, Lin X, Wen K, Zhu J, He H. Effects of organic templates on the structural properties of porous clay heterostructures: a non-micellar template model for porous structure. J Porous Mater. 2015;22:219–28.
Breen C. Thermogravimetric study of the desorption of cyclohexylamine and pyridine from an acid-treated Wyoming bentonite. Clay Miner. 1991;26:473–86.
Bagshaw SA, Cooney RP. FTIR surface site analysis of pillared clays using pyridine probe species. Chem Mater. 1993;5:1101–9.
Lambert JF, Poncelet G. Acidity in pillared clays: origin and catalytic manifestations. Top Catal. 1997;11:43–56.
Wei L, Tang T, Huang B. Novel acidic porous clay heterostructure with highly ordered organic–inorganic hybrid structure: one-pot synthesis of mesoporous organosilica in the galleries of clay. Microporous Mesoporous Mater. 2004;67:175–9.
Cavani F, Guidetti S, Marinelli L, Piccinini M, Chedini E, Signoretto M. The control of selectivity in gas-phase glycerol dehydration to acrolein catalysed by sulfated zirconia. Appl Catal B. 2010;100:197–204.
Carriazo D, Domingo C, Martin C, Rives V. PMo or PW heteropoly acids supported on MCM-41 silica nanoparticles: characterisation and FT-IR study of the adsorption of 2-butanol. J Soild State Chem. 2008;181:2046–57.
Ma Y, Sun H, Sun Q, Zhang H. Zirconium-doped porous magadiite heterostructures upon 2D intragallery in situ hydrolysis–condensation–polymerization strategy for liquid-phase benzoylation. RSC Adv. 2015;5:67853–65.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kooli, F., Liu, Y., Hbaieb, K. et al. Factors that affect the thermal stability and properties of Zr-porous clay heterostructures. J Therm Anal Calorim 126, 1143–1155 (2016). https://doi.org/10.1007/s10973-016-5825-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5825-8