Abstract
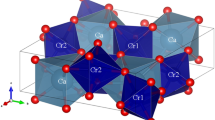
The thermal expansions of three kinds of α-CL-20 crystals have been investigated in the temperature range from 30 to 130 °C by means of various-temperature X-ray powder diffraction (XRD) together with Rietveld refinement. The crystals were characterized by SEM, XRD, FTIR, and DSC/TG. The results show that the three samples contain 1/2, 1/4 mol, and none structural water, respectively. The α-CL-20 crystals all perform linear and anisotropic thermal expansion, while some differences exist. The unit-cell axes increase linearly with increasing temperature except for the a-axis. The expansion along the a-axis switches from positive to negative thermal expansion at 90 °C for α-CL-20·1/2H2O. The a-axis exhibits positive thermal expansion (PTE) with thermal irresilience for α-CL-20·1/4H2O while PTE with thermal resilience for the anhydrous α-CL-20. The differences are caused by the structural water. The removal of structural water leads to the collapse of a-axis, further results in structural changes of the unit cell. Different water contents can cause different degree of structural changes, leading to the difference of thermal expansion behaviors.










Similar content being viewed by others
References
Gialanella S, Marino F. Effect of microstructure on thermal expansion behaviour of nanocrystalline metallic materials. J Mater Sci Technol. 2010;45(3):824–30.
El-Mallawany R, Abdel-Kader A, El-Hawary M, El-Khoshkhany N. Volume and thermal studies for tellurite glasses. J Mater Sci Technol. 2010;45(4):871–87.
Radhakrishnan A, Prabhakar Rao P, Mahesh S, Vaisakhan Thampi D, Koshy P. Role of bond strength on the lattice thermal expansion and oxide ion conductivity in quaternary pyrochlore solid solutions. Inorg Chem. 2012;51(4):2409–19.
Kovalevsky AV, Yaremchenko AA, Populoh S, Weidenkaff A, Frade JR. Effect of a-site cation deficiency on the thermoelectric performance of donor-substituted strontium titanate. J Phys Chem C. 2014;118(9):4596–606.
Sikora M, Bernatowicz P, Szafrański M, Katrusiak A. Quasistatic disorder of NH···N bonds and elastic-properties relationship in 2-phenylimidazole crystals. J Phys Chem C. 2014;118(13):7049–56.
Miller W, Smith C, Mackenzie D, Evans K. Negative thermal expansion: a review. J Mater Sci Technol. 2009;44(20):5441–51.
Lim T-C. Negative thermal expansion structures constructed from positive thermal expansion trusses. J Mater Sci Technol. 2012;47(1):368–73.
Nicolaï B, Rietveld IB, Barrio M, Mahé N, Tamarit JL, Céolin R, Guéchot C, Teulon JM. Uniaxial negative thermal expansion in crystals of tienoxolol. Struct Chem. 2013;24(1):279–83.
Michael BJ, Catherine AW, Mary AW. Negative thermal expansion materials. J Therm Anal Calorim. 2010;99(1):165–72.
Bhattacharya S, Saha BK. Steric guided anomalous thermal expansion in a dimorphic organic system. Cryst Eng Comm. 2014;16(12):2340–3.
Kroll P, Andrade M, Yan X, Ionescu E, Miehe G, Riedel R. Isotropic negative thermal expansion in β-Si (NCN)2 and its origin. J Phys Chem C. 2011;116(1):526–31.
Weese RK, Burnham AK. Coefficient of thermal expansion of the beta and delta polymorphs of HMX. Propellants Explos Pyrotech. 2005;30(5):344–50.
Leiber CO. Assessment of safety and risk with a microscopic model of detonation. Amsterdam: Elsevier; 2003. p. 524.
Skidmore C, Butler T, Sandoval C. The elusive coefficients of thermal expansion in PBX 9502. NM (US): Los Alamos National Lab; 2003.
Kolb JR, Rizzo H. Growth of 1, 3, 5-triamino-2, 4, 6-trinitrobenzene (TATB) I. anisotropic thermal expansion. Propellants Explos Pyrotech. 1979;4(1):10–6.
Herrmann M, Engel W, Eisenreich N. Thermal expansion, transitions, sensitivities and burning rates of HMX. Propellants Explos Pyrotech. 1992;17(4):190–5.
Novikov VV, Avdashchenko DV, Matovnikov AV, Mitroshenkov NV, Bud SL. Heat capacity and thermal expansion of icosahedral lutetium boride LuB66. J Therm Anal Calorim. 2014;116(1):765–9.
Novikov VV, Mitroshenkov NV, Matovnikov AV, Avdashchenko DV, Trubnickov SV, Morozov AV. Peculiarities of the lattice thermal properties of rare-earth tetraborides. J Therm Anal Calorim. 2015;102(2):1597–602.
Litasov KD, Gavryushkin PN, Yunoshev AS, Rashchenko SV, Inerbaev TM, Akilbekov AT. Thermal expansion of coronene C24H12 at 185-416 K. J Therm Anal Calorim. 2015;119(11):1183–9.
Sahu M, Krishnan K, Saxena MK, Dash S. Combustion synthesis and thermal expansion of RE6UO12 (RE = Dy and Tb). J Therm Anal Calorim. 2013;112(1):165–72.
Samui P, Gupta K, Dash S, Dahale ND, Naik Y. Thermoluminescence and linear thermal expansion of MgAl2O4. J Therm Anal Calorim. 2014;115(10):1289–94.
Ivanov MG, Shmakov AN, Drebushchak VA, Podyacheva OY. Two mechanisms of thermal expansion in perovskite SrCo0.6Fe0.2Nb0.2O3−z. J Therm Anal Calorim. 2010;110(6):79–82.
Sun J, Shu X, Liu Y, Zhang H, Liu X, Jiang Y, Kang B, Xue C, Song G. Investigation on the thermal expansion and theoretical density of 1, 3, 5-trinitro-1, 3, 5-triazacyclohexane. Propellants Explos Pyrotech. 2011;36(4):341–6.
Xue C, Sun J, Kang B, Liu Y, Liu X. The b-d-phase transition and thermal expansion of octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine. Propellants Explos Pyrotech. 2009;35:333–8.
Shu X, Tian Y, Song G, Zhang H, Kang B, Zhang C, Liu Y, Liu X, Sun J. Thermal expansion and theoretical density of 2,2′,4,4′,6,6′-hexanitrostilbene. J Mater Sci Technol. 2011;46(8):2536–40.
Sun J, Kang B, Xue C, Liu Y, Xia Y, Liu X, Zhang W. Crystal state of 1,3,5-triamino-2,4,6-trinitrobenzene (TATB) undergoing thermal cycling process. Cent Eur J Energ Mater. 2010;28(3):189–201.
Sun J, Kang B, Zhang H, Liu Y, Xia Y, Yao Y, Liu X. Investigation on irreversible expansion of 1,3,5-triamino-2,4,6-trinitrobenzene cylinder. Cent Eur J Energ Mater. 2011;8(1):69–79.
Evers J, Klapötke TM, Mayer P, Oehlinger G, Welch J. α-and β-FOX-7, polymorphs of a high energy density material, studied by X-ray single crystal and powder investigations in the temperature range from 200 to 423 K. Inorg Chem. 2006;45(13):4996–5007.
Kempa P, Herrmann M. Temperature resolved X-ray diffraction for the investigation of the phase transitions of FOX-7, Part. Part Syst Charact. 2005;22(6):418–22.
Gump JC, Stoltz CA, Mason BP, Freedman BG, Ball JR, Peiris SM. Equations of state of 2,6-diamino-3,5-dinitropyrazine-1-oxide. J Appl Phys. 2011;110(7):073523.
Geetha MNU, Sarwade D, et al. Studies on CL-20:the most powerful high energy material. J Therm Anal Calorim. 2003;73(3):913–22.
Agrawal JP. Recent trends in high-energy materials. Prog Energy Combust Sci. 1998;24(1):1–30.
Simpson R, Urtiew P, Ornellas D, Moody G, Scribner K, Hoffman D. CL-20 performance exceeds that of HMX and its sensitivity is moderate. Propellants Explos Pyrotech. 1997;22(5):249–55.
Foltz MF, Coon CL, Garcia F, Nichols AL. The thermal stability of the polymorphs of hexanitrohexaazaisowurtzitane, Part I. Propellants Explos Pyrotech. 1994;19(1):19–25.
Xu J, Sun J, Zhou K. Review on polymorphic transformation of CL-20 in recrystallization. Chin J Energ Mater. 2012;20(2):248–55.
Xu J, Tian Y, Liu Y, Zhang H, Shu Y, Sun J. Polymorphism in hexanitrohexaazaisowurtzitane crystallized from Solution. J Cryst Growth. 2012;354:13–9.
Xu J, Pu L, Liu Y. Polymorphic transformation of ε-CL-20 in different HTPB-based composite systems. Chin J Energ Mater. 2015;23(2):113–9.
Qi LY, Zeman S, Svoboda R, Elbeih A. The effect of crystal structure on the thermal reactivity of CL-20 and its C4-bonded explosives. J Therm Anal Calorim. 2013;112(9):837–49.
Zhang P, Xu JJ, Guo XY, Jiao QJ, Zhang JY. Effect of addictives on polymorphic transition of ε-CL-20 in castable systems. J Therm Anal Calorim. 2014;110(6):79–82.
Sorescu DC, Rice BM, Thompson DL. Molecular packing and NPT-molecular dynamics investigation of the transferability of the RDX intermolecular potential to 2, 4, 6, 8, 10, 12-hexanitrohexaazaisowurtzitane. J Phys Chem B. 1998;102(6):948–52.
Nadezhda B, Bolotina M. J H R L. Energetic materials:variable-temperature crystal structures of γ- and ε-HNIW polymorphs. J Appl Crystallogr. 2004;37:808–14.
Gump JC, Peiris SM. Phase transitions and isothermal equations of state of epsilon hexanitrohexaazaisowurtzitane (CL-20). J Appl Phys. 2008;104(8):083509.
Diffracplus Topas (version 3.0) User’s manual. Axs Bruker Inc: Madsion, WI; 2005.
Engel W, Fraunhofer. Inst.f. chemische Technologie ICT. Pfinaztal, Germany, ICDD. Grant-in-Aid; 2005.
Foltz MF, Coon CL, Garcia F, Nichols AL. The thermal stability of the polymorphs of hexanitrohexaazaisowurtzitane, Part II. Propellants Explos Pyrotech. 1994;19(3):133–44.
Li J, Brill TB. Kinetics of solid polymorphic phase transitions of CL-20. Propellants Explos Pyrotech. 2007;32(4):326–30.
Acknowledgements
This study was financially supported by the Youth Talent Training Foundation of Institute of Chemical Materials (QNRC-201205), the National Natural Science Foundation of China (11472252, 11372290), and the Foundation of China Academy of Engineering Physics (2012A0201007).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pu, L., Xu, J., Song, G. et al. Investigation on the thermal expansion of α-CL-20 with different water contents. J Therm Anal Calorim 122, 1355–1364 (2015). https://doi.org/10.1007/s10973-015-4884-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4884-6