Abstract
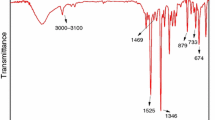
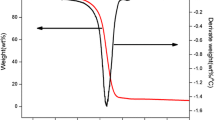
A phosphorous flame retardant of phosphoric acid, P, P′-1, 4-phenylene P, P, P′, P′-tetraphenyl ester (PAPTE) was synthesized by diphenyl chlorophosphate and hydroquinone. The structural features of PAPTE were investigated with mass spectrometry, infrared spectroscopy, X-ray powder diffraction and nuclear magnetic resonance methods. The thermal behavior of PAPTE was carried out with thermogravimetric (TG) analysis instruments. The kinetic parameters, including the activation energy and pre-exponential factor of the decomposition process for the title compound, were calculated through the Friedman method, the Kissinger–Akahira–Sunose method and the Flynn–Wall–Ozawa method, while the thermal decomposition mechanism was also studied with the invariant kinetic parameters (IKP) method based on a set of TG data obtained at different heating rates. It was shown that the activation energies calculated for the decomposition reaction by different methods were found to be consistent with the conversion functions calculated by means of the IKP method which depend on a set of kinetic methods.










Similar content being viewed by others
References
Bao X, Cai XF. Synergistic effect of methyl phenyl silicone resin and DOPO on the flame retardancy of epoxy resins. J Therm Anal Calorim. 2014;118:369–75.
Zheng JY, Li X, Yu YJ, et al. Novel high phosphorus content phosphaphenanthrene-based efficient flame retardant additives for lithium-ion battery. J Therm Anal Calorim. 2014;117:319–24.
Richardson N, Dellar RJ. Flame-resistant polymer compositions containing salts of phosphonic acids. Eur patent 0,245,207. 1992.
Fan FQ, Xia ZB, Li QY, et al. Thermal stability of phosphorus-containing styrene-acrylic copolymer and its fire retardant performance in waterborne intumescent coatings. J Therm Anal Calorim. 2013;114:937–46.
Schmelzer HG, Schmidt M. Yeager TP. Thermoplastic ternary molding composition of polyurethane polyphosphonates and polycarbonate resins. US patent 4,350,799. 1982.
Wang N, Wu YH, Mi L, et al. The influence of silicone shell on double-layered microcapsules in intumescent flame-retardant natural rubber composites. J Therm Anal Calorim. 2014;118:349–57.
Yuan DD, Yin HQ, Cai XF. Synergistic effects between silicon-containing flame retardant and potassium-4-(phenylsulfonyl)benzenesulfonate (KSS) on flame retardancy and thermal degradation of PC. J Therm Anal Calorim. 2013;114:19–25.
Chen Q, Chen R, Huang B, et al. Preparation of flame retardant polyolefin composition comprises mixing ammonium polyphosphate, 2,4,6-tri(N,N-dihydroxy ethyl)amino-1,3,5-triazine and nitrogen and phosphorus-containing branched polymer and extruding with polyolefin resin. CN patent 102,660,059. 2012.
Wu B, Wang YZ, Wang XL, et al. Kinetics of thermal oxidative degradation of phosphorus-containing flame retardant copolyesters. Polym Degrad Stab. 2002;76:401–9.
Asrar J, Berger PA, Hurlbut J. Synthesis and characterization of a fire-retardant polyester: copolymers of ethylene terephthalate and 2-carboxyethyl (phenylphosphinic) acid. J Polym Sci Pol Chem. 1999;37:3119–28.
Joseph P, Ebdon JR. Phosphorus-Based Flame Retardants (Chapter 5). In: Wilkie CA, Morgan AB, editors. Fire Retardancy of Polymeric Materials. CRC Press, Boc a Raton, 2010. p. 107–127.
Gao M, Wu WH, Liu S, et al. Thermal degradation and flame retardancy of rigid polyurethane foams containing a novel intumescent flame retardant. J Therm Anal Calorim. 2014;117:1419–25.
Chen YZ, Peng HQ, Li JH, et al. A novel flame retardant containing phosphorus, nitrogen, and sulfur. J Therm Anal Calorim. 2014;115:1639–49.
Imanishi S, Okumura Y. Plasticizers containing phosphate ester, and cellulose ester compositions with good fire resistance and thermoplasticity. JP Patent 0,076,057. 2013.
Yang L, Fu QJ, Wang XL, et al. Method for preparing organic phosphate series flame retardant with p-benzenediol phosphate as skeleton. CN Patent 102,617,635. 2012.
Stowell J, Levchik SV. Phosphate ester-treated flame retarded textiles, production methods and articles. WO Patent 2,012,061,373. 2012.
Friedman HL. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J Polym Sci Part C Polym Symp. 1964;6(1):183–95.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Zhu FL, Feng QQ, Xu YF, et al. Kinetics of pyrolysis of ramie fabric wastes from thermogravimetric data. J Therm Anal Calorim. 2015;119:651–7.
Flynn JH, Wall LA. General treatment of the thermogravimetry of polymers. J Res Natl Bur Stand A. 1966;70:487–523.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881–6.
Lesnikovich AI, Levchik SV. A method of finding invariant values of kinetic parameters. J Therm Anal Calorim. 1983;27:89–93.
Lesnikovich AI, Levchik SV. Isoparametric kinetic relations for chemical transformations in condensed substances (analytical survey). Reactions involving the participation of solid substances. J Therm Anal Calorim. 1985;30:677–702.
Vyazovkin S, Burnham AK, Criado JM, et al. ICTAC kinetics committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520:1–19.
Tang WJ, Liu YW, Zhang H, et al. New approximate formula for Arrhenius temperature integral. Thermochim Acta. 2003;408:39–43.
Richard-Campisi L, Bourbigot S, Bras ML, et al. Thermal behaviour of cotton-modacrylic fibre blends: kinetic study using the invariant kinetic parameters method. Thermochim Acta. 1996;275:37–49.
Garcìa-Pèrez M, Chaala A, Yang J, et al. Co-pyrolysis of sugarcane bagasse with petroleum residue. Part 1. Thermogravimetric analysis. Fuel. 2001;80:1245–58.
Vyazovkin S, Linert W. Kinetic analysis of reversible thermal decomposition of solids. Int J Chem Kinet. 1995;27:73–84.
Twarowskd A. Reduction of a phosphorus oxide and acid reaction set. Combust Flame. 1995;102:41–54.
Macdonald MA, Gouldin FC, Fisher EM. Temperature dependence of phosphorus-based flame inhibition. Combust Flame. 2001;124:668–83.
Han YQ, Li TX, Saito K. Comprehensive method based on model free method and IKP method for evaluating kinetic parameters of solid state reactions. J Comput Chem. 2012;33:2516–25.
Conflict of interest
The authors declare no competing financial interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, GM., Wang, LS., Sun, J. et al. Synthesis and thermal kinetic study of phosphoric acid, P, P′-1, 4-phenylene P, P, P′, P′-tetraphenyl ester. J Therm Anal Calorim 122, 349–358 (2015). https://doi.org/10.1007/s10973-015-4678-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4678-x