Abstract
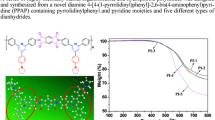
The synthesis and thermal research of a pyridine-containing polyimide were reported in this work. The corresponding diamine monomer, 2,6-bis(4-(4-aminophenoxy)phenyl)-4-phenyl pyridine (BApPP), was synthesized through combining the nucleophilic aromatic substitution with modified Chichibabin reaction and hydrazine monohydrate reduction. Fourier transform infrared spectroscopy (FT-IR), 1H nuclear magnetic resonance spectroscopy (1H-NMR), and elemental analysis were used for chemical structural characterization, the results identified the chemical structures of the monomer and polymer. The melting behavior of the diamine monomer was studied by differential scanning calorimetry (DSC). The thermal properties of the resulting polyimide were investigated from DSC and thermo gravimetric analysis together. The results indicated the resulting polyimide exhibited excellent thermal stability, with the glass transition temperature around 275.0 °C and the initial mass loss of sample beyond 487.5 °C. The thermal decomposition process was analysed by combining the Avrami–Erofeev dynamic model and Ozawa–Flynn–Wall method. The coefficients of the thermal decomposition kinetics equation were calculated, and the final equation was obtained by polynomial fitting method.












Similar content being viewed by others
References
Liaw DJ, Wang KL, Huang YC, Lee KR, Lai JY, Ha CS. Advanced polyimide materials: syntheses, physical properties and applications. Prog Polym Sci. 2012;37:907–74.
Baek JB, Qin H, Mather PT, Tan LS. A new hyperbranched poly (arylene-ether-ketone-imide): synthesis, chain-end functionalization, and blending with a bis (maleimide). Macromolecules. 2002;35:4951–9.
Hu Z, Yin Y, Kita H, Okamoto KI, Suto Y, Wang H, Kawasato H. Synthesis and properties of novel sulfonated polyimides bearing sulfophenyl pendant groups for polymer electrolyte fuel cell application. Polymer. 2007;48:1962–71.
Khazaka R, Locatelli M, Diaham S, Bidan P. Effects of mechanical stresses, thickness and atmosphere on aging of polyimide thin films at high temperature. Polym Degrad Stab. 2013;98:361–7.
Luo L, Pang Y, Jiang X, Wang X, Zhang P, Chen Y, Peng C, Liu X. Preparation and characterization of novel polyimide films containing amide groups. J Polym Res. 2012;19:1–7.
Wang DH, Riley JK, Fillery SP, Durstock MF, Vaia RA, Tan LS. Synthesis and characterization of unsymmetrical benzonitrile-containing polyimides: viscosity-lowering effect and dielectric properties. J Polym Sci Polym Chem. 2013;51:4998–5011.
Wang X, Li Y, Ma T, Zhang S, Gong C. Synthesis and characterization of novel polyimides derived from 2, 6-bis [4-(3, 4-dicarboxyphenoxy) benzoyl] pyridine dianhydride and aromatic diamines. Polymer. 2006;47:3774–83.
Yamanaka K, Jikei M, Kakimoto M. Synthesis of hyperbranched aromatic polyimides via polyamic acid methyl ester precursor. Macromolecules. 2000;33:1111–4.
Chang Y-H, Wu M-S, Lin K-F. Graphting polyimide to MWCNT for enhancing dispersion and properties of MWCNT/polyetherimide nanocomposites. J Polym Res. 2014;21:1–7.
Yamanaka K, Jikei M, Kakimoto M. Preparation and properties of hyperbranched aromatic polyimides via polyamic acid methyl ester precursors. Macromolecules. 2000;33:6937–44.
Yamanaka K, Jikei M, Kakimoto M. Preparation of hyperbranched aromatic polyimide without linear units by end-capping reaction. Macromolecules. 2001;34:3910–5.
Çakmakçı E, Güngör A. Preparation and characterization of flame retardant and proton conducting boron phosphate/polyimide composites. Polym Degrad Stab. 2013;98:927–33.
Xia S, Yi L, Sun Z, Wang Y. The effect of phthalimide side chains on the thermal stability and rubbing resistance of polyimide used as a liquid crystal vertical alignment layer. J Polym Res. 2013;20:1–12.
Chang YT, Shu CF. Synthesis of hyperbranched aromatic poly (amide-imide): copolymerization of B’B2 monomer with A2 monomer. Macromolecules. 2003;36:661–6.
Chen YC, Lo WC, Juang TY, Dai SA, Su WC, Chou CC, Jeng RJ. Thermally stable hyperbranched nonlinear optical polyimides using an “A2 + B3” approach. Mater Chem Phys. 2011;127:107–13.
Hao J, Jikei M, Kakimoto M. Synthesis and comparison of hyperbranched aromatic polyimides having the same repeating unit by AB2 self-polymerization and A2 + B3 polymerization. Macromolecules. 2003;36:3519–28.
Dixit BC, Dixit RB, Desai DJ. Synthesis and characterization of novel ion-exchange resin based on polyimide containing 8-hydroxyquinoline as a pendent groups. J Polym Res. 2010;17:481–8.
Ghosh A, Sen SK, Banerjee S, Voit B. Solubility improvements in aromatic polyimides by macromolecular engineering. RSC Adv. 2012;2:5900–26.
Kim SD, Kim SY, Chung IS. Soluble and transparent polyimides from unsymmetrical diamine containing two trifluoromethyl groups. J Polym Sci Polym Chem. 2013;51:4413–22.
Shao Y, Li Y, Zhao X, Ma T, Gong C, Yang F. Synthesis and characterization of soluble polyimides derived from a novel unsymmetrical diamine monomer: 1, 4-(2′, 4″-diaminodiphenoxy) benzene. Eur Polym J. 2007;43:4389–97.
Xia S, Sun Z, Yi L, Wang Y. Synthesis of soluble polyimide derived from novel naphthalene diamines for liquid crystal alignment layers and a preliminary study on the mechanism of imidization. RSC Adv. 2013;3:14661–70.
Zeng K, Guo Q, Gao S, Wu D, Fan H, Yang G. Studies on organosoluble polyimides based on a series of new asymmetric and symmetric dianhydrides: structure/solubility and thermal property relationships. Macromol Res. 2012;20:10–20.
Zhuo L, Kou K, Wang Y, Yao P, Wu G. Synthesis of soluble and thermally stable polyimides with phthalimide as pendent group from pyridine-containing triamine. J Mater Sci. 2014;49:5141–50.
Vyazovkin S. Kinetic concepts of thermally stimulated reactions in solids: a view from a historical perspective. Int Rev Phys Chem. 2000;19:45–60.
Vyazovkin S, Sbirrazzuoli N. Isoconversional kinetic analysis of thermally stimulated processes in polymers. Macromol Rapid Commun. 2006;27:1515–32.
Blanco I, Abate L, Bottino FA. Synthesis and thermal properties of new dumbbell-shaped isobutyl-substituted POSSs linked by aliphatic bridges. J Therm Anal Calorim. 2014;116:5–13.
Koga N. Ozawa’s kinetic method for analyzing thermoanalytical curves. J Therm Anal Calorim. 2013;113:1527–41.
Othman MBH, Ramli R, Ariff ZM, Akil HM, Ahmad Z. Thermal properties of polyimide system containing silicone segments. J Therm Anal Calorim. 2012;109:1515–23.
Šesták J, Šimon P, Holba P. Hot topics of thermal analysis. J Therm Anal Calorim. 2013;114:459–62.
Zhang J-Q, Gao H-X, Ji T-Z, Chao M, Ma H-X, Xu K-Z, Hu R-Z. Synthesis and thermal behaviors of 1, 3-bis (4-aminophenoxy) benzene (TPER) and polyimide based on TPER and pyromellitic dianhydride. J Therm Anal Calorim. 2013;114:441–9.
Burnett JF, Zahler RE. Aromatic nucleophilic substitution reactions. Chem Rev. 1951;49:273–412.
Tamami B, Yeganeh H. Preparation and properties of novel polyimides derived from 4-aryl-2,6 bis (4-amino phenyl) pyridine. J Polym Sci Polym Chem. 2001;39:3826–31.
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC kinetics committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520:1–19.
Acknowledgements
The authors are grateful to the National Natural Science Foundation of China (No. 51407134) and the graduate starting seed fund of Northwestern Polytechnical University (No. Z2014175).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhuo, L., Kou, K., Yao, P. et al. Preparation and thermal decomposition kinetics research of pyridine-containing polyimide. J Therm Anal Calorim 119, 2039–2051 (2015). https://doi.org/10.1007/s10973-014-4360-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-4360-8