Abstract
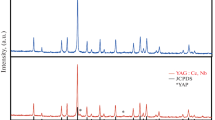
Different types of precursors were prepared by gel combustion starting from yttrium–cerium nitrate, TEOS, and different fuels (i.e., threonine, arginine, citric acid, and glycine). The precursors were fired at 1400 °C in nitrogen or air atmosphere in order to obtain cerium-activated (Y2SiO5:Ce) phosphors with X2-monoclinic structure. The processes involved during the heating of precursors were put in evidence using thermal analysis investigations (TG–DTG–DTA). Correlation between the thermal decomposition steps, mass loss, and composition of evolved gases during the thermal treatment was established using TG/DTA–FT-IR coupling. Precursors prepared with threonine, arginine, and glycine have a similar thermal behavior with a mass loss up to 10.0 %, due to the decomposition of hydrated carbonates. Unreacted nitrate compounds or organic residues have been identified when combustion occurs in fuel-lean (<0.79 mol threonine) or in fuel-rich conditions (>0.79 mol threonine), respectively. Citric acid forms citrate-based compounds during gelation process with inhibitory effect on the combustion process. The conversion to well-crystallized silicates was revealed by changes of FT-IR vibration bands and confirmed by XRD measurements. A blue emission with variable intensities was observed for all phosphor samples depending on the amount and fuel type. Combustion with threonine leads to phosphors with highest luminescent characteristics.










Similar content being viewed by others
References
Ronda CR. Luminescence: from theory to applications. 1st ed. Weinheim: Wiley; 2008.
Ropp RC. Luminescence and solid State. 2nd ed. Amsterdam: Elsevier Science; 2004.
Kalaycioglu NO, Cırcır E. Red phosphors in MgAl2Si2O8 doping with Mn4+, Gd3+ and Lu3+ and host-sensitized luminescence properties. J Therm Anal Calorim. 2013;111:273–7.
Korosin NC, Francetic V, Bukovec N. Thermal and luminescent properties of Eu2+ doped aluminates prepared by the sol–gel method. J Therm Anal Calorim. 2013;111:1291–6.
Li C, Wyon C, Moncorge R. Spectroscopic properties and fluorescence dynamics of Er3+ and Yb3+ in Y2SiO5. IEEE J Quantum Electron. 1992;28:1209–21.
Peters TE. Cathodoluminescent Ln y (SiO2) x :Tb Phosphors. J Electrochem Soc. 1969;116:985–9.
Denoyer A, Levesque Y, Jandl S, Goldner P, Guillot-Noel O, Viana B, Thibault F, Pelenc D. Cooperative emission study in ytterbium-doped Y2SiO5. J Lumin. 2008;128:1389–93.
Bosze EJ, Hirata GA, Mckittrick J. An investigation of the chromaticity of blue emitting yttrium silicate. MRS Symp Proc. 1999;560:15–20.
Busta HH. Field emission flat displays. In: Zhu W, editor. Vacuum microelectronics. New York: Wiley; 2001. p. 289–343.
Jiao H, Wang XJ, Ye S, Jing XP. Morphology of Gd3+-doped Y2SiO5:Ce. J Lumin. 2007;122–123:113–6.
Wang YZ, He QY, Chu BL. Synthesis and characterization of Ce-doped Lu2SiO5 powders by the solid-state reaction with Li2SO4 flux. J Alloys Compd. 2009;479:704–6.
Zhang ZH, Wang YH, Hao Y, Liu WJ. Synthesis and VUV photoluminescence of green-emitting X2–Y2SiO5:Tb3+ phosphor for PDP application. J Alloys Compd. 2007;433:L12–4.
Jiao H, Wei L, Zhang N, Zhong M, Jing X. Melting salt assisted sol–gel synthesis of blue phosphor Y2SiO5:Ce. J Eur Ceram Soc. 2007;27:185–9.
Lee HJ, Hong SK, Jung DS, Ju SH, Koo HY, Kang YC. The characteristics of X1 type Y2SiO5:Tb phosphor particles prepared by high temperature spray pyrolysis. Ceram Int. 2006;32:865–70.
Cooke DW, Lee J-K, Bennett BL, Groves JR, Jacobsohn LG, McKigney EA, Muenchausen RE, Nastasi M, Sickafus KE, Tang M, Valdez JA. Luminescent properties and reduced dimensional behavior of hydrothermally prepared Y2SiO5:Ce nanophosphors. Appl Phys Lett. 2006;88:103108.
Bosze EJ, McKittrick J, Hirata GA. Investigation of the physical properties of a blue-emitting phophor produced using a rapid exothermic reaction. Mat Sci Eng B. 2003;97:265–74.
Patil KC, Hegde MS, Rattan T, Aruna ST. Chemistry of nanocrystalline oxide materials. Singapore: World Scientific Publishing Co., Pvt. Ltd.; 2008.
Aruna ST, Mukasyan AS. Combustion synthesis and nanomaterials. Curr Opin Solid State Mater Sci. 2008;12:44–50.
McKittrick J, Shea LE, Bacalski CF, Bosze EJ. The influence of processing parameters on luminescent oxides produced by combustion synthesis. Displays. 1999;19:169–72.
Bhatkar VB, Omanwar SK, Moharil SV. Combustion synthesis of silicate phosphors. Opt Mater. 2007;29:1066–70.
Onani MO, Dejene FB. Photo-luminescent properties of a green or red emitting Tb3+ or Eu3+ doped calcium magnesium silicate phosphors. Phys B. 2014;439:137–40.
Rakov N, Maciel GS. Three-photon up-conversion and optical thermometry characterization of Er3+:Yb3+ co-doped yttrium silicate powders. Sens Actuators B. 2012;164:96–100.
Gonzalez-Ortega JA, Tejeda EM, Perea N, Hirata GA, Bosze EJ, McKittrick J. White light emission from rare earth activated yttrium silicate nanocrystalline powders and thin films. Opt Mater. 2005;27:1221–7.
Kulkarni S, Nagabhushana BM, Suriyamurthy N, Shivakumarad C, Chakradhar RPS, Damle R. Synthesis, luminescence and EPR studies on CaSiO3:Pb, Mn-nano phosphors synthesized by the solution combustion method. Ceram Int. 2013;39:1917–22.
Mostafavi K, Ghahari M, Baghshahi S, Arabi AM. Synthesis of Mg2SiO4:Eu3+ by combustion method and investigating its luminescence properties. J Alloys Compd. 2013;555:62–7.
Muenchausen RE, McKigney EA, Jacobsohn LG, Blair MW, Bennett BL, Cooke DW. Science and application of oxyorthosilicate nanophosphors. IEEE Trans Nucl Sci. 2008;55:1532–5.
Yukihara EG, Jacobsohn LG, Blair MW, Bennett BL, Tornga SC, Muenchausen RE. Luminescence properties of Ce-doped oxy orthosilicate nanophosphors and single crystals. J Lumin. 2010;130:2309–16.
Ramakrishna G, Nagabhushana H, Sunitha DV, Prashantha SC, Sharma SC, Nagabhushana BM. Effect of different fuels on structural, photo and thermo luminescence properties of solution combustion prepared Y2SiO5 nanopowders. Spectrochim Acta A. 2014;127:177–84.
Muresan LE, Oprea BF, Cadis AI, Perhaita I, Ponta O. Studies on Y2SiO5:Ce phosphors prepared by gel combustion using new fuels. J Alloys Compd. 2014;615:795–803.
D’Assuncao LM, Giolito I, Ionashiro M. Thermal decomposition of the hydrated basic carbonates of lanthanides and yttrium. Thermochim Acta. 1989;137:319–30.
Zhang QY, Pita K, Ye W, Que WX, Kam CH. Effects of composition and structure on spectral properties of Eu3+-doped yttrium silicate transparent nanocrystalline films by metallorganic decomposition method. Chem Phys Lett. 2002;356:161–7.
Ismail HM, Hussein GAM. Texture properties of yttrium oxides generated from different inorganic precursors. J Powder Technol. 1996;87:87–92.
Yongiu L, Xiaoyun L, Yizheng W, Junming L, Weili S. Preparation and characterization of porous yttrium oxide powders with high specific surface area. J Rare Earths. 2006;24:34–8.
Pop N, Mogos AM, Vlase G, Vlase T, Doca N. Theoretic analysis and experimental evidence for relationships between the derivative thermogravimetric curves and the Gramm–Schmidt profiles. J Therm Anal Calorim. 2013;113:113–9.
Portela Marques MM, Miranda Salvado IM, Margac FMA, Ferreira LM. The role of zirconium as thermal stabilizer of PDMS–TEOS hybrids. J Therm Anal Calorim. 2010;100:557–61.
Nakamoto K. Infrared and Raman Spectra of inorganic and coordination compounds. 4th ed. New York: Wiley; 1986.
Zhecheva RSE, Gorova M, Alcántara R, Morales J, Tirado JL. Lithium–cobalt citrate precursors in the preparation of intercalation electrode materials. Chem Mater. 1996;8:1429–40.
Todorovsky DS, Getsova MM, Wawer I, Stefanov P, Enchev V. On the chemical nature of lanthanum-titanium citric complexes, precursors of La2Ti2O7. Mater Lett. 2004;58:3559–63.
Manlian H, Kai G, Zhenyong M, Haohong C, Xinxin Y, Fangfang X, Jingtai Z. Morphology controllable synthesis of yttrium oxide-based phosphors from yttrium citrate precursors. J Rare Earths. 2011;29:830–6.
Narendar Y, Messing GL. Synthesis, decomposition and crystallization characteristics of peroxo–citrato–niobium: an aqueous niobium precursor. Chem Mater. 1997;9:580–7.
Stoia M, Barvinschi P, Barbu-Tudoran L. Thermal decomposition of metal nitrates PVA–TEOS gels for obtaining M(II) ferrite/silica nanocomposites. J Therm Anal Calorim. 2013;113:21–30.
Draegert DA, Stone NWB, Curnutte B, Williams D. Far-infrared spectrum of liquid water. J Opt Soc Am. 1966;56:64–9.
Smith BC. Infrared spectral interpretation a schematic approach. Boca Raton: CRC Press; 1999.
Aitasalo T, Holsa J, Lastusaari M, Legendziewicz J, Niittykoski J, Pelle F. Delayed luminescence of Ce3+ doped Y2SiO5. Opt Mater. 2004;26:107–12.
Acknowledgements
This work was supported by a Grant of the Romanian National Authority for Scientific Research, CNCS – UEFISCDI, project number PN-II-RU-TE-2012-3-0360.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Muresan, L.E., Cadis, A.I., Perhaita, I. et al. Thermal behavior of precursors for synthesis of Y2SiO5:Ce phosphor via gel combustion. J Therm Anal Calorim 119, 1565–1576 (2015). https://doi.org/10.1007/s10973-014-4315-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-4315-0