Abstract
Borate-doped silicate glasses with chemical compositions of (70 − x)SiO2–xB2O3–30CaO (x = 0, 5, 15, and 25, in mol%) were synthesized using the sol–gel method, intended to be used in tissue regeneration. The effects of borate content on the glass surface morphology, chemical structure, ion dissolution behavior, and fibroblast compatibility were investigated. 11B magic angle spinning-solid state nuclear magnetic resonance and Fourier transform infrared spectra demonstrated that borate, in the glasses, possessed both three- and four-coordinated structures. From nitrogen sorption, the specific surface area of the glasses decreased with increased borate content and calcination temperature, from 600 °C to 700 °C. In the case of glasses undergoing calcination at 700 °C, silicate and calcium ion released in a Tris–HCl buffer solution (pH = 7.4) at the early stage of the immersion test decreased as borate content increased. The decrease in surface area caused by stabilizing at 700 °C due to the effect of increasing borate concentration controlled the ion dissolution behavior of the glasses. The proliferation ability of fibroblasts cultured with the dissolution products of the glasses were improved as borate content increased in the glass composition.
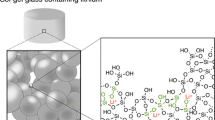
Graphical Abstract

Borosilicate bioactive glasses were developed using a sol–gel method and effects of borate content on the glass surface morphology, chemical structure, ion dissolution behavior, and fibroblast compatibility were investigated. We found that four-coordinated units of borate were formed, the specific surface area of the glasses decreased with increased borate content and calcination temperature, and silicate and calcium ion release in a buffer solution was controlled by borate-doping.
Highlights
-
Borate-doped silicate glasses were synthesized using the sol–gel method.
-
The SSA of the glasses decreased with increased borate content and calcination temperature.
-
Silicate and calcium ion release in a buffer solution was controlled by borate-doping.
-
The proliferation ability of fibroblasts was improved after treated with borate-doped glasses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Sol–gel derived glasses have attracted attention in the field of biomaterials. Li et al. [1] developed the ternary sol–gel bioactive glass (BG) 58 S (60SiO2–36CaO–4P2O5, in mol%) and then Saravanapavan et al. [2] simplified it into the binary 70S30C composition (70SiO2–30CaO, in mol%). Because of their characteristic nanoporous structure, sol–gel BGs have higher ion release ability compared with those BGs fabricated via the melt–quenching method. The nanopores enhance the specific surface area [2], and therefore, sol–gel BGs have more rapid degradation rates than melt–quenching–derived BGs of similar composition [1]. Sol–gel BG scaffolds with unique structures have been developed, and these structures can expand the application fields of BGs. For example, mesoporous glasses and cotton–wool-like structured scaffolds with flexibility are expected to be good candidates for not only bone regeneration but also local drug/protein delivery systems, skin regeneration, etc [3,4,5,6,7,8].
Because several kinds of inorganic ions have been found to stimulate cells, such as osteoblasts and mesenchymal stem cells, to proliferate, differentiate, etc., sol–gel BGs are expected to be more versatile tools to investigate tissue regeneration through stimulation by ions [9,10,11,12]. However, to achieve a potentially accelerated tissue regeneration, a controlled ion release from sol–gel BGs is needed because most cell reactions depend on the amounts of ions provided to the cells. An initial burst of ion release from BGs and the subsequent pH increase provoke undesirable reactions, such as cell death and inflammation. Midha et al. [13] reported that porous 70S30C scaffolds induced inflammatory responses in a rat when they were implanted without any preconditioning (incubation in a cell culture medium for 3 days prior to implantation) and they suggested that the inflammatory responses were due to increased pH in the region local to the scaffolds. They also reported in another paper that preconditioned 70S30C scaffolds could promote vascularized bone regeneration by supporting mineralization of preosteoblasts, vascularization of endothelial cells, and resorption by osteoclasts [14]. Therefore, 70S30C scaffolds after preconditioning, i.e., scaffolds without any initial burst of ions, would be an ideal material for tissue regeneration, although logistically difficult to implement in a clinical setting. In addition, most calcium ions and a large amount of silicate ions are released from sol–gel BGs during the preconditioning process, but these ions are necessary to promote cell functions and tissue regeneration. Sol–gel BGs that can be used without preconditioning are needed.
Borate glasses can form three-coordinated (BO3) and four-coordinated (BO4) units and exhibit nonlinear properties controlled by the amount of network modifier cation, termed “the borate anomaly”. Network modifier addition initially increases glass network connectivity by forming four-coordinated (BO4) units and then the connectivity decreases upon further network modifier additions [15, 16]. Similar phenomena have also been seen for borosilicate glasses [17]. Structural change related to boron coordination is known to influence the physicochemical properties of glasses, such as chemical durability. For example, the chemical durability of melt–quench-derived borate glasses, such as the B2O3-Li2O system, has been reported to vary with the ratio of lithium to borate with nonlinear dependence. In a particular range of the ratio of lithium to borate, the chemical durability increased by adding lithium because the connectivity was increased by forming BO4 in the glass [18]. Thus, although borate glasses have inherently lower network connectivity, their chemical durability could be controlled by forming BO4.
Several reports on borate and borosilicate glasses prepared using sol–gel methods have been published [19,20,21,22,23,24,25,26], but their number is smaller than that of melt–quench-derived borate glasses. This might be because of the difficulty in gel formation for sol–gel borate glasses. In addition, the origin of the borate anomaly of sol–gel borate and borosilicate glasses is still unclear. To the best of our knowledge, the only report on the borate anomaly in sol–gel borate glasses is that by Lepry et al. [21]. They studied the physicochemical properties of borate glasses, xCaO–(100 − x)B2O3 (x = 20–70 mol%) and clarified changes in glass structures, texture, and hydroxyapatite-forming ability in simulated body fluid in accordance with the borate anomaly. The properties of sol–gel borosilicate glasses are also expected to vary in accordance with the change in boron coordination, which might contribute to suppression of the initial burst of ion release from the glass. However, borate has been introduced into silicate-based BGs for the purpose of decreasing their chemical durability, regardless of the synthesis method of the glasses (i.e., sol–gel or melt–quenching) [26,27,28]. Tohamy et al. [26] introduced borate into 70SiO2–24CaO–6P2O5 (mol%) sol–gel glass and expected lower chemical durability and higher ability of hydroxyapatite formation in the simulated body fluid. Conversely, Deilmann et al. [29] reported that boron incorporation increases the bulk modulus and hardness of mesoporous BG with 80SiO2–15CaO–5P2O5 (mol%), whereas no increase in chemical durability was found. In other silicate sol–gel BGs, borate has also been added to silicate-based systems as a modifier [22, 25, 26, 30,31,32], but there are no reports on their enhanced chemical durability or suppressed initial bursts of ion release.
Boron/borate ions have been attracting attention in the wound-healing fields because they promote proliferation and differentiation of dermal and stem cells, angiogenesis, and formation of extracellular matrix [33,34,35]. Furthermore, dietary boron can also stimulate tissue regeneration, including bone [36, 37]. Therefore, we expected that introducing an appropriate amount of borate into 70S30C would achieve not only suppression of the ion release behavior but also improved ability to promote tissue regeneration. Herein, we synthesized sol–gel borosilicate glasses based on 70S30C and characterized their glass structure, especially borate networks and glass surface topography. The ion release behavior of the glasses in Tris-buffered solution was characterized to see the effect of borate doping on the initial ion burst. Finally, fibroblast proliferation with dissolution products of the glasses was evaluated.
2 Materials and methods
2.1 Synthesis of SiO2–CaO and SiO2–B2O3–CaO sol–gel glasses
Binary and ternary glasses with compositions of (70 − x)-mol% SiO2, x-mol% B2O3, and 30-mol% CaO (x = 0, 5, 15, 25) were synthesized using the sol–gel method. Sample compositions and codes are shown in Table 1. To prepare the sols, distilled water (DW), ethanol (EtOH, 99.9%, Wako Pure Chemical Industries, Ltd.), and 2 N nitric acid (HNO3) were mixed for 0.5 h in a PTFE container. The silicate precursor (tetraethylorthosilicate, TEOS; 98.0%, Sigma-Aldrich) was added to the resulting solution dropwise and mixed for 1 h. The borate precursor (triethylborate, TEB; 99%, Sigma-Aldrich) was also added dropwise and mixed for 1 h. The ratio of TEOS + TEB: DW: EtOH: HNO3 was 1:3:2:0.05 (mol). The calcium precursor (calcium nitrate tetrahydrate, Ca(NO3)2・4H2O; 98.5%, Wako Pure Chemical Industries, Ltd.) was added to the resulting solution and mixed for 1 h in a closed container. The aforementioned process was performed under nitrogen atmosphere.
The closed container containing the resulting solution was placed in an oven and heat treated for gelation at 60 °C for 72 h. The obtained gels were dried using a three-step program (60 °C for 20 h, 90 °C for 24 h, and 130 °C for 20 h, ramp = 0.1 °C/min) and then calcined using a two-step program (300 °C for 2 h and 600 °C or 700 °C for 5 h, ramp = 1 °C/min). The obtained glasses were pulverized, and the resulting powders were sieved to obtain powders 32–125 µm in size.
2.2 Characterization
2.2.1 Nitrogen sorption
Samples were degassed at 150 °C for 2 h in a vacuum prior to measurement to remove physically adsorbed gases, especially water vapor, from the sample surface. Nitrogen sorption was conducted for 20 adsorption and 20 desorption points with a Belsorp-mini II (MicrotracBEL, Japan) (n = 1). Specific surface area (SSA) was obtained using the Brunauer–Emmett–Teller (BET) [38] equation. The Barrett–Joyner–Halenda (BJH) method [39], using the adsorption isotherms [40], provided average pore width and pore volume.
2.2.2 X-ray diffraction
X-ray diffraction (XRD, X’Pert X-ray Diffractometer, Phillips) analysis was conducted (Cu Kα, 45 kV, 40 mA). Diffraction patterns were collected for 2θ values between 6° and 50° with a step speed of 0.21 °/sec and a step size of 0.1017°. Samples were placed on an amorphous silicon disk during measurement.
2.2.3 Fourier transform infrared spectroscopy
Attenuated total reflectance-Fourier transform infrared (FTIR, FTIR-4000, JASCO, Japan), using a diamond crystal, was performed on the samples. An air background was measured before measuring the samples. Spectra were recorded from 600 to 1800 cm−1 with a resolution of 4.0 cm−1 and average of 128 scans.
2.2.4 Magic angle spinning-solid state nuclear magnetic resonance spectroscopy
11B single pulse magic angle spinning (MAS)-solid state nuclear magnetic resonance (NMR) experiments were performed on an JNM-ECA600II (JEOL) operating at a resonance frequency of 192.56 MHz. The spectrometers were equipped with a 3.2-mm ZrO2 probe, which enables implementation of a MAS frequency of 20 kHz. Flip angle calibration was conducted on sodium tetrahydroborate from which a π/2 pulse time of 0.1 μs was measured. All measurements were conducted with a π/2 tip angle along with a recycle delay between subsequent pulses of 50 s. Gaussian function fitting was performed for the obtained spectra.
2.3 Dissolution study
Glass samples were immersed in Tris–HCl buffer solutions (TBS, pH 7.4) at a glass-to-media ratio of 1.5 mg/mL [41]. A quantity of 75 mg of glass powder was placed in a polyethylene container with a screw cap. To this, 50 mL of TBS was added to start the dissolution test. After sealing the container, the immersed samples were kept in an incubator shaker at 37 °C with a rotation speed of 120 rpm. A quantity of 0.5 mL of solution was collected at 4, 8, 24, 48, and 72 h after the incubation. The elemental concentrations of silicon, boron, and calcium of the collected solution were determined using inductively coupled plasma atomic emission spectrometry (ICP-AES, ICPS-7510, Shimadzu, Japan). The sample solutions for ICP-AES were prepared by diluting the collected solution (0.5 mL) 20 times with DW. Standards of silicon, boron, and calcium were prepared at 0, 5, 10, 20, and 40 ppm for calibration. All samples were run in triplicate for statistical analysis. TBS alone was incubated under the same conditions and used as a control.
2.4 Cell culture test
Mouse fibroblast-like cell lines (NIH 3T3) were seeded at a density of 5 × 104 mL−1 in 96-well plates with DMEM media (Gibco, USA) supplemented with 10% fetal bovine serum (Gibco, USA) and 1% penicillin/streptomycin (Gibco, USA). After culturing the cells for 3 h in a humidified incubator with 5% CO2, the media were removed, and 100 μL of the extracts of each glass sample was added in the 96-well plate. The extracts were prepared by immersing 45 mg of glass powder in 10 mL of the cell culture medium (4.5 mg/mL) and then maintaining them for 24 h in an incubator shaker (37 °C, 120 rpm), followed by filtration with a 0.2-μm filter. Only culture medium was used as the “control” sample. The media in each well were changed every other day. The number of cells in the 96-well plates was counted by using alamarBlue (Invitrogen) and following instructions for the kit. Briefly, 110 µL of alamarBlue solution (after being diluted 10 times using the culture medium) was added in each well and left for 4 h in the CO2 incubator. A quantity of 80 µL of solution from each well was moved to another well plate, and then the metabolic activity of the cells in each well was estimated by measuring fluorescence with an excitation wavelength of 540 nm and emission wavelength of 590 nm using a multimode plate reader (EnSpire, PerkinElmer) (n = 4). The statistical significance was determined using the Dunnett test (p < 0.05).
3 Results and discussion
3.1 Surface and textural properties
The physisorption of nitrogen on the samples was mainly monolayer formation followed by saturation of the fibers on the surface, leading to type IV isotherms, except for 15B 700 and 25B 700 (type III), as shown in Fig. 1. The materials categorized as type III are non-porous materials and materials that have weak interaction between the adsorbate and adsorbent. Type IV corresponds to mesoporous materials [40]. Table 2 shows the SSA and mean pore diameter of prepared glass powders with different calcination temperatures. The SSA values decreased with the increase in B2O3 content in the glass. In particular, this phenomenon is obvious for the glasses after calcination at 700 °C; the value drastically decreases for 15B and 25B. After calcining at 700 °C, the SSA of the samples decreased because of further crosslinking of the B2O3 and SiO2 networks under viscous flow of the glass during sintering. The pore size of 25B 700 was no longer measurable using the BJH method due to low adsorption of N2; i.e., there were no pores present, or they were smaller than 2 nm.
Tohamy et al. [26] showed that the glass transition temperature (Tg) and crystallization temperature (Tc) of B2O3–substituted SiO2–CaO–P2O5 sol–gel glasses decreased as the amount of B2O3 increased. Thus, the decreased SSA for the B2O3-doped samples after calcination at 700 °C, especially 15B/25B 700, resulted from changes in surface topography due to shrinking because of their lower sintering temperature.
3.2 Chemical structure
Figure 2 shows XRD patterns of 0B, 5B, 15B, and 25B calcined at 600 °C and 700 °C. Regardless of the composition and calcination temperature, broad halo peaks were observed at 2θ = 15–35°. Although 0B exhibited a halo peak at approximately 25°, the borate-doped samples also exhibited another halo peak at ~28°. For 0B 600/700, 5B 600/700, and 15B 700, small peaks at 26.9°, 29.2°, 32.6°, and 41.1° were identified as β-wollastonite [42] and β-Ca2SiO4 [43]. Crystallization decreased as borate content increased.
For the FTIR spectra (Fig. 3), the two main regions associated with borate were present at 850–1200 cm−1 (B–O stretching of BO4 units) and 1200–1500 cm−1 (B–O stretching of BO3 units) [19, 44]. The intensity of the peaks corresponding to BO3 increased with an increase in the amount of B2O3 doped in the glass. Such a phenomenon was unclear for BO4 because the peak region for BO4 overlaps that of Si–O bonds. The main band of silicate-based glass was present at 800–1200 cm−1, especially the peaks at approximately 850, 950, 1050, and 1200 cm−1 derived from the Si–O bond of QSi0, QSi2, QSi3, and QSi4, respectively. From the XRD and FTIR characterization results, all samples predominantly consisted of amorphous phases, whereas some crystalline phases were present in 0B 600/700, 5B 600/700, and 15B 700. This indicates that the doped B2O3 suppressed the crystallization of the glass. Similar phenomena were observed for materials reported by Ciceo et al. [30] and Tohamy et al. [26].
Four types of samples, 5B 600/700 and 25B 600/700, were studied using 11B MAS-NMR spectroscopy (Fig. 4). The NMR spectra allow us to distinguish BO3 from BO4 units through separated chemical sift regions (from 5 to 20 ppm and from −5 to 3 ppm for BO3 and BO4, respectively) [45]. The abundance ratio of BO4 in the borate network can be described using the fraction of four-coordinated boron, N4 [46]:
Here, B3 and B4 denote the amount of three- and four-coordinated boron, respectively, which are obtained by measurement of the peak area. There was almost no difference between samples calcined at 600 °C: N4 was 0.55 and 0.56 (Fig. 4a). The samples calcined at 700 °C possess smaller N4: values for 15B 700 and 25B 700 were 0.42 and 0.49, respectively (Fig. 4b). In addition, the difference in N4 between 15B and 25B was larger for samples calcined at 700 °C compared with those calcined at 600 °C.
Tanaka et al. [17] investigated the relation between glass basicity and N4 degree of borosilicate glass. Glass basicity, Λ, is expressed as follows in below equations [17] as
where Zi is the oxidation number of the cation I and ri is the ionic ratio with respect to the total number of oxides. γi in Eqs. (2) and (3) is the basicity moderating parameter and is empirically given by the Pauling electronegativity, χi. For borosilicate glasses, N4 has been reported to reach a maximum when Λ is in the range of 0.50–0.55 [17]. Because the Λ values for 5B, 15B, and 25B are 0.56, 0.55, and 0.54, respectively, 15B and 25B are expected to exhibit the maximum N4. The N4 values decreased with an increase in calcination temperature, from 600 to 700 °C. This might indicate that calcium ions penetrate the borate network at higher temperature, which then induces the change from BO4 to BO2O− (boron with two bridging oxygen and one non-bridging oxygen). A larger amount of calcium ions is expected to penetrate the borate network for 15B in comparison with 25B because the N4 values decreased proportionately more as the calcination temperature increased from 600 to 700 °C. This would be involved in the borate anomaly, but further studies are needed to elucidate the reasons why such phenomena occurred.
3.3 Ion release from glasses in TBS
Ion release from glass particles was evaluated up to 72 h in TBS. Although simulated body fluid and phosphate buffer solution are also popular as solutions used for immersion tests for biomaterials, TBS was used in this study because it contains no ions related to the precipitates (e.g., hydroxyapatite and calcite). It is possible to measure each ion amount dissolved from the samples in a buffer solution.
The concentration profiles of silicon, calcium, and boron upon immersion in TBS are shown in Fig. 5. Silicate dissolution behavior of the samples calcined at 600 °C (Fig. 5a) indicated almost no difference, showing a rapid increase in concentration within 8 h and then a gradual increase. Conversely, the behavior of samples calcined at 700 °C (Fig. 5a’) changed with the amount of doped B2O3. As the composition ratio of B2O3 increased, the silicate release at the early stage of immersion (~8 h) was suppressed. For example, after immersion for 8 h, the silicon concentration of 0B 700 and 25B 700 was about 37 ppm and 12 ppm, respectively. The silicon concentration of 15B 700 and 25B 700 increased steadily from 12–13 ppm to 41–48 ppm until reaching the final time point (72 h) The release behavior of calcium (Fig. 5b, b’) exhibited a similar pattern to that of silicate: a large amount of calcium, 223–259 ppm, was released from the samples calcined at 600 °C within 8 h, and then, the release plateaued. In contrast, for 15B 700 and 25B 700, calcium ions maintained a gradual release until 72 h. Borate release behavior (Fig. 5c, c’) was different from the other two elements. The amount of released borate increased as the content of B2O3 in the glasses increased, from 5B through 15B to 25B, and this trend was similar with a change in the calcination temperature. The release behavior of ions, especially silicate and calcium, from the glass particles was therefore controlled best through a change in B2O3 content when the calcination temperature was 700 °C. In particular, the dissolution of 15B and 25B 700 was more sustained over the immersion time, compared to other compositions and lower stabilization temperature, where dissolution was less linear and more of a burst release. The suppression of calcium release from the glasses must contribute to the control of pH because, in aqueous solutions, pH change follows the exchange of calcium ions from glasses with H+/H3O+ from the surrounding solution [47]. In addition, the suppression of calcium release is expected to influence the hydroxyapatite forming ability of the glasses in simulated body fluid and bioactivity in the body. Evaluation of such behavior of the glasses will be performed in a future study.
The solubility of glasses in aqueous solutions predominantly relates to the chemical composition and surface morphology of the glasses. In general, sol–gel-derived glasses are more soluble compared with glasses derived from the melt–quenching method due to their nanoporosity [1, 2]. Sol–gel glasses have higher specific surface area, whereas melt–quenching-derived glasses possess a dense structure. For example, in the present study, 0B exhibited a rapid ion release within 8 h after immersion, which is regarded as being typical of the behavior of sol–gel glasses. Conversely, suppression of silicate and calcium release was observed for 15B 700 and 25B 700, which is a result of their decreased SSA, as shown in Table 2.
Although the interaction between the BO4 unit and charge-compensating calcium ions was another possible cause for suppression, it must not be the predominant cause. This is because 15B 600 and 25B 600 contain a large amount of BO4, as shown in Fig. 4. However, no difference was found in the ion release behavior between all samples calcined at 600 °C, including 0B. Even if an interaction between the BO4 unit and the charge-compensating calcium ions formed in 15B 600 and 25B 600, it would be a weaker Coulombic interaction. Therefore, in the case of samples calcined at 600 °C, their large SSA should contribute to their initial burst of silicate and calcium release.
3.4 NIH 3T3 cell responses
Cytocompatibility was assessed by measuring the metabolic activity of NIH 3T3 cultured with the dissolution products of the glasses. The results of alamarBlue assay shown in Fig. 6 demonstrated that 0B 700 and 5B 700 suppressed proliferation of the cells compared to the control. According to the immersion test results for these samples (Fig. 5b, b’), a large amount of calcium ions would be immediately released from the glasses and exchanged with protons from the culture medium, which must lead to a rapid increase in pH. This higher pH is expected to result in deteriorating cell activity [13]. Conversely, in the case of 15B 700 and 25B 700, the cells kept growing over 7 days and there was no significant difference compared with the control at Days 3 and 5. This could be due to two factors: (1) a smaller change in the pH due to suppressed calcium ion release and (2) positive effects of dissolved borate ions on cell functions [33,34,35]. Thus, the calcium borosilicate sol–gel-derived glasses may be more effective for enhancement of cell activities and subsequent tissue regeneration in the body compared with calcium silicate glasses.
4 Conclusions
Borosilicate BGs were prepared using the sol–gel method, and their structural and dissolution properties and cell viability were evaluated. A higher composition ratio of borate and higher calcination temperature (700 °C) induced a reduction in pore size on sample surfaces, which contributed to decreased SSA. In addition, these samples exhibited a gradual and more sustained release of silicate and calcium ions in TBS, without an initial burst of the release. From cell culture tests using fibroblasts, these samples possessed higher cell metabolic activity than those with just silicate glass (glass containing no borate). This is because the borosilicate glasses exhibited a controlled initial burst of the ion release, especially the calcium ion release, which might lead to a subsequent stable pH and/or positive effects of dissolved borate ions on cell functions. Thus, it is possible to control the ion release behavior and cell compatibility of calcium silicate BGs by incorporating appropriate amounts of borate into the glass composition and treating with higher calcination temperatures. These novel borosilicate glasses are expected to be good candidates for materials used in tissue regeneration. The slower ion release, compared to silicate-only glass, suggests that the material could potentially reduce (or even eliminate) an inflammatory response without compromising on performance.
References
Li R, Clark AE, Hench LL (1991) J Appl Biomater 2:231–239. https://doi.org/10.1002/jab.770020403
Saravanapavan P, Jones JR, Pryce RS, Hench LL (2003) J Biomed Mater Res Part A 66A:110–119. https://doi.org/10.1002/jbm.a.10532
Jones JR (2013) Acta Biomater 9:4457–4486. https://doi.org/10.1016/j.actbio.2012.08.023
López-Noriega A, Arcos D, Izquierdo-Barba I, Sakamoto Y, Terasaki O, Vallet-Regí M (2006) Chem Mat 18:3137–3144. https://doi.org/10.1021/cm060488o
Hartmann M (2005) Chem. Mat. 17:4577–4593. https://doi.org/10.1021/cm0485658
Norris E, Ramos-Rivera C, Poologasundarampillai G, Clark JP, Ju Q, Obata A, Hanna JV, Kasuga T, Mitchell CA, Jell G, Jones JR (2020) Biomed Mater 15:015014. https://doi.org/10.1088/1748-605x/ab591d
Saha S, Bhattacharjee A, Rahaman SH, Ray S, Marei MK, Jain H, Chakraborty J (2020) Int J Appl Glass Sci 11:320–328. https://doi.org/10.1111/ijag.15029
Qin X, Cao R, Zheng J, Shi G, Ji L, Zhu A, Yao H (2020) RSC Adv. 10:44835–44840. https://doi.org/10.1039/D0RA08656H
Hoppe A, Güldal NS, Boccaccini AR (2011) Biomaterials 32:2757–2774. https://doi.org/10.1016/j.biomaterials.2011.01.004
Xynos ID, Edgar AJ, Buttery LDK, Hench LL, Polak JM (2000) Biochem Biophys Res Commun 276:461–465. https://doi.org/10.1006/bbrc.2000.3503
Jones JR, Tsigkou O, Coates EE, Stevens MM, Polak JM, Hench LL (2007) Biomaterials 28:1653–1663. https://doi.org/10.1016/j.biomaterials.2006.11.022
Naruphontjirakul P, Tsigkou O, Li S, Porter AE, Jones JR (2019) Acta Biomater 90:373–392. https://doi.org/10.1016/j.actbio.2019.03.038
Midha S, Kim TB, van den Bergh W, Lee PD, Jones JR, Mitchell CA (2013) Acta Biomater 9:9169–9182. https://doi.org/10.1016/j.actbio.2013.07.014
Midha S, van den Bergh W, Kim TB, Lee PD, Jones JR, Mitchell CA (2013) Adv Healthc Mater 2:490–499. https://doi.org/10.1002/adhm.201200140
Uhlmann DR, Shaw RR (1969) J Non-Cryst Solids 1:347–359. https://doi.org/10.1016/0022-3093(69)90018-0
Priven AI (2000) Glass Phys Chem 26:441–454. https://doi.org/10.1007/BF02732065
Tanaka Y, Benino Y, Nanba T, Sakida S, Miura Y (2009) Phys Chem Glasses-B 50:289–293
Veléz MH, Tuller HL, Uhlmann DR (1982) J Non-Cryst Solids 49:351–362. https://doi.org/10.1016/0022-3093(82)90131-4
Lepry WC, Nazhat SN (2015) Chem Mat 27:4821–4831. https://doi.org/10.1021/acs.chemmater.5b01697
Lepry WC, Smith S, Nazhat SN (2018) J Non-Cryst Solids 500:141–148. https://doi.org/10.1016/j.jnoncrysol.2018.07.042
Lepry WC, Nazhat SN (2020) Mater Adv. 1:1371–1381. https://doi.org/10.1039/D0MA00360C
Kumar A, Mariappan CR, Sarahan BS (2019) J Non-Cryst Solids 505:431–437. https://doi.org/10.1016/j.jnoncrysol.2018.11.024
Tohge N, Mackenzie JD (1984) J Non-Cryst Solids 68:411–418. https://doi.org/10.1016/0022-3093(84)90021-8
Bengisu M, Yilmaz E, Farzad H, Reis ST (2008) J Sol-Gel Sci Tech 45:237–243. https://doi.org/10.1007/s10971-008-1681-8
Seyedmomeni SS, Naeimi M, Raz M, Mohandesi JA, Moztarzadeh F, Baghbani F, Tahriri M (2018) Silicon 10:197–203. https://doi.org/10.1007/s12633-016-9414-z
Tohamy KM, Soliman IE, Motawea AE, Aboelnasr MA (2015) Nat Sci 13:145–154
Huang W, Day DE, Kittiratanapiboon K, Rahaman MN (2006) J Mater Sci Mater Med 17:583–596. https://doi.org/10.1007/s10856-006-9220-z
Schuhladen K, Pantulap U, Engel K, Jeleń P, Olejniczak Z, Hupa L, Sitarz M, Boccaccini AR (2021) Int J Appl Glass Sci. 12:293–312. https://doi.org/10.1111/ijag.15894
Deilmann L, Winter O, Cerrutti B, Bradtmüller H, Herzig C, Limbeck A, Lahayne O, Hellmich C, Eckert H, Eder D (2020) J Mater Chem B 8:1456–1465. https://doi.org/10.1039/C9TB01805K
Lucacel Ciceo R, Trandafir D-L, Radu T, Ponta O, Simon V (2014) Ceram Int. 40:9517–9524. https://doi.org/10.1016/j.ceramint.2014.02.025
Thomas BJC, Shaffer MSP, Boccaccini AR (2009) Compos Pt A-Appl Sci Manuf 40:837–845. https://doi.org/10.1016/j.compositesa.2009.04.006
Trandafir DL, Ponta O, Ciceo-Lucacel R, Simon V (2015) J Mol Struct 1080:111–116. https://doi.org/10.1016/j.molstruc.2014.09.065
Demirci S, Doğan A, Aydın S, Dülger EÇ, Şahin F (2016) Mol Cell Biochem 417:119–133. https://doi.org/10.1007/s11010-016-2719-9
Lin Y, Brown RF, Jung SB, Day DE (2014) J Biomed Mater Res Part A 102:4491–4499. https://doi.org/10.1002/jbm.a.35120
Zhou J, Wang H, Zhao S, Zhou N, Li L, Huang W, Wang D, Zhang C (2016) Mater Sci Eng C 60:437–445. https://doi.org/10.1016/j.msec.2015.11.068
Nielsen FH (2008) Nutr Rev 66:183–191. https://doi.org/10.1111/j.1753-4887.2008.00023.x
Dzondo-Gadet M, Mayap-Nzietchueng R, Hess K, Nabet P, Belleville F, Dousset B (2002) Biol Trace Elem Res 85:23–33. https://doi.org/10.1385/BTER:85:1:23
Brunauer S, Deming LS, Deming WE, Teller E (1940) J Am Chem Soc 62:1723–1732. https://doi.org/10.1021/ja01864a025
Barrett EP, Joyner LG, Halenda PP (1951) J Am Chem Soc 73:373–380. https://doi.org/10.1021/ja01145a126
Sing KSW (1985) Pure Appl Chem 57:603–619. https://doi.org/10.1351/pac198557040603
Maçon ALB, Kim TB, Valliant EM, Goetschius K, Brow RK, Day DE, Hoppe A, Boccaccini AR, Kim IY, Ohtsuki C, Kokubo T, Osaka A, Vallet-Regí M, Arcos D, Fraile L, Salinas AJ, Teixeira AV, Vueva Y, Almeida RM, Miola M, Vitale-Brovarone C, Verné E, Höland W, Jones JR (2015) J Mater Sci Mater Med 26:115. https://doi.org/10.1007/s10856-015-5403-9
Lin K, Lin C, Zeng Y (2016) RSC Adv 6:13867–13872. https://doi.org/10.1039/C5RA26916D
Dai Y, Liu H, Liu B, Wang Z, Li Y, Zhou G (2015) Ceram Int 41:5894–5902. https://doi.org/10.1016/j.ceramint.2015.01.021
Manupriya, Thind KS, Singh K, Kumar V, Sharma G, Singh DP, Singh D (2009) J Phys Chem 70:1137–1141. https://doi.org/10.1016/j.jpcs.2009.05.025
Doris M, Gregory T, Anja W-B, Lothar W, Efstratios IK (2015) Phys Chem Glasses-B 56:203–211. https://doi.org/10.13036/17533562.56.5.203
Wu J, Stebbins JF (2014) J Am Ceram Soc 97:2794–2801. https://doi.org/10.1111/jace.13100
Cerruti M, Greenspan D, Powers K (2005) Biomaterials 26:1665–1674. https://doi.org/10.1016/j.biomaterials.2004.07.009
Funding
This work was supported by JSPS KAKENHI Grant Number 18K12075 and 20H00304, Nippon Sheet Glass Foundation for Materials Science and Engineering and the Hibi Science Foundation.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by DI. ALBM, and AO. The first draft of the manuscript was written by AO and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ishihara, D., Maçon, A.L.B., Norris, E. et al. Borosilicate sol–gel bioactive glasses and the effect of borate content on structure-property relationships. J Sol-Gel Sci Technol (2023). https://doi.org/10.1007/s10971-023-06075-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10971-023-06075-0