Abstract
The development of low-temperature sol–gel (solution) processes for the fabrication of crystalline metal oxide thin films has become a key objective in the emerging Flexible Electronics. To achieve this target, crystalline oxide films need to be deposited on flexible substrates, which have degradation temperatures below 350 °C (e.g., polymers or textile). This achievement would be a step towards improving the performance of the flexible device, making feasible applications now restrained (e.g. smart-skin, flexible-displays or solar-cells) and whose performance is associated to the functional properties of the crystalline oxide (e.g., ferroelectricity, pyroelectricity or piezoelectricity). However, this is a challenge because the crystallization of these oxides usually occurs at high temperatures, over 600 °C. This paper shows an overview to the solution strategies devised in our group for the low-temperature fabrication of crystalline metal oxide thin films, mostly ferroelectric perovskites (e.g., BiFeO3, PbTiO3 or Pb(Zr,Ti)O3). We have made use of UV-light as an alternative energy source to the thermal energy conventionally used to obtain the crystalline oxide. High photosensitive sol–gel solutions have been synthesized and the solution-deposited layers irradiated with UV-excimer lamps. A precise control of the photoreactions occurring during the irradiation of these layers has been carried out with the aim of advancing the formation of a high-densified, defect-free amorphous metal oxide film that easily can be converted into crystalline at temperatures compatible with the use of polymer substrates.
Graphical Abstract

An overview to the solution strategies devised in our group for the low-temperature fabrication of crystalline metal oxide thin films, mostly ferroelectric perovskites, is shown in this article. UV-light is used as an alternative energy source to the conventional thermal energy used to obtain crystalline oxide thin films. These solution strategies are based in the synthesis of high photosensitive sol–gel solutions and the irradiation of the solution-deposited layers with UV-excimer lamps under controlled irradiation atmospheres. A precise control of the photoreactions occurring in the system during the irradiation process has been carried out, with the aim of advancing the formation of high-densified, defect-free amorphous metal oxide films able to be converted into crystalline at temperatures compatible with the use of polymer substrates. This makes possible the integration of these advanced materials in the emerging Flexible Electronics.
Highlights
-
Low-temperature sol–gel (solution) methods for the fabrication of crystalline metal oxide films.
-
UV-light-assisted Chemical Solution Deposition of films for accelerating the oxide crystallization.
-
Thin film crystallization at temperatures ≤350 °C on flexible polyimide substrates.
-
Functional properties of crystalline metal oxide films on flexible polyimide substrates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Today, the electronic industry is demanding cost efficient, soft-portable, and high-tech devices. This has pushed the advance of Flexible Electronics, where the active layer is mainly supported on cheap flexible plastics with degradation temperatures ≤400 °C (e.g., polyimides (PI), which are the plastics with the highest glass transition temperatures) [1]. To satisfy these consumer needs, efforts have been made to fabricate high-performance crystalline metal oxide thin films on flexible substrates [2]. However, crystallization conventionally requires high temperatures, over 600 °C. Therefore, the incorporation of crystalline metal oxides in flexible devices has been pushing research on alternative fabrication processes that permit the crystallization of functional films at processing temperatures compatible with the use of flexible substrates [2,3,4].
In addition, flexible electronic devices not only calls for low-temperature fabrication processes but also for deposition techniques that scale easily to the sizes of flexible devices. Among the different film deposition techniques, solution methods are the best positioned to meet these demands [2,3,4]. Achieving these targets would provide additional benefits, such as the development of sustainable solution fabrication processes with low-energy consumption and reduced carbon footprint [5,6,7,8].
Nowadays, amorphous metal oxide semiconductors are the most widely used materials in Flexible Electronics because they require relatively low processing temperatures. However, most of these solution-processed films require annealing temperatures over 350 °C, due to the inherent oxide formation mechanism and the need to remove any organic residue [1]. Therefore, the development of low-temperature solution approaches has been driven by the direct integration of such amorphous oxides with Flexible Electronics.
Figure 1 shows some of the most successful solution approaches developed for the low-temperature fabrication of amorphous metal oxide films [6], They are mainly based on the design of novel solution precursors and/or the use of innovative post-treatment methods. A more difficult challenge is attaining the crystallization of the metal oxide film, because it occurs several hundred degrees above the degradation temperatures of polymer substrates. Some of these low-temperature solution methods have been previously tested for the preparation of crystalline metal oxide thin films on plastics [3, 4]. However, attaining an ordered array of atoms in the film with a low content of defects and porosity is essential to achieve not only crystallinity in the film but also appropriate physical properties, which ultimately define their functionality (e.g., ferroelectricity, piezoelectricity, superconductivity, etc.). This is a challenge for achieving a successful integration of crystalline metal oxide films with flexible substrates in functional devices [2]. In this regard, different methods have also been developed for transferring crystalline films prepared on single-crystal substrates at high temperatures into flexible substrates [3, 4, 9]. However, these procedures require the use of thermal treatments at high temperatures to obtain the primary crystalline film, and, in general, are not so efficient in the fabrication of large-area and flexible devices as it does occur in the direct deposition of the film by low-temperature solution deposition methods.
Schematic diagram of low-temperature approaches for solution-processed oxide thin film transistors. Reprinted with permission from ref. [6], Copyright © 2018 American Chemical Society
During the last years, our group has carried out an ambitious investigation in the field of the low-temperature processing of crystalline metal oxide thin films by Chemical Solution Deposition (CSD) methods. These studies were initially inspired in the need of fabricating ferroelectric perovskite films on semiconductor substrates at temperatures below 500 °C, which is the maximum limit imposed by the microelectronic integration routines [10]. Furthermore, we have also been encouraged by the knowledge accumulated in the field of the flexible semiconductor metal oxide films, thus trying to break the 350 °C barrier that makes possible the direct solution deposition of crystalline metal oxide films on flexible plastic substrates [1, 6]. We have made a deep investigation about the use of UV-light sources for the low-temperature crystallization of metal oxide thin films, which is presented as an alternative energy source to the traditional thermal heating [11]. This processing strategy successfully developed by our group for the fabrication of functional crystalline films at low temperatures is inspired in the pioneering works of the Imai’s group on the fabrication of sol–gel materials assisted by UV-irradiation [12, 13].
In the next section, we will describe the effects of using continuous UV-light irradiation at different stages of the CSD processing of metal oxide thin films to reduce their crystallization temperatures to those compatible with the use of flexible polyimide substrates.
2 Low-temperature solution processing of metal oxide thin films
The fabrication of crystalline metal oxide thin films from solutions is traditionally divided in five stages: (i) solution synthesis, (ii) solution deposition, (iii) decomposition of the metal precursors contained in the solution-deposited layer, (iv) condensation and compaction of the amorphous film, and (v) film crystallization [5].
The sol(solution)-gel precursors (as-deposited amorphous layers) always have a large amount of organic compounds and the only way of accelerating the nucleation and growth of the crystal phase is the quick elimination of these organics. This results in the formation of an interconnected metal oxygen (–M–O–M–) network, which is the building block of the crystalline metal oxide material. If we are able to minimize the content of defects and to obtain a high-densified amorphous metal oxygen layer, then the energy of activation (Ea) barrier, which needs to be surpassed for the film crystallization, is reduced and a lower amount of thermal energy would be required to reach the ordered atomic arrangement in the crystalline film (Fig. 2). Therefore, all the low-temperature solution strategies reported in the literature for the fabrication of crystalline metal oxide films are directed towards the fulfilling of this principle.
Evolution of the free energy as a function of temperature during the crystallization of amorphous metal oxide films prepared by chemical solution deposition (CSD) methods. a Schematic diagram of the free energies of a solution-derived amorphous film and the crystalline perovskite phase (ΔGV = driving force for oxide crystallization). Inset shows the energy activation barrier, Ea, that should be surpassed for the amorphous to crystalline conversion of the metal oxide. b Scheme showing the three major steps to obtain crystalline thin films from solutions, starting from a solution layer with alarge content of organics, porosity and carbonaceous residues, which is converted into a high-densified, defect-free amorphous metal oxide film and then into a crystalline film with an ordered array of the metal and oxygen atoms
In the next section, a detailed revision of the most successful low-temperature solution methods of functional crystalline thin films devised in our group is shown. Most of them make use of continuous UV-irradiation of the deposited solution layer to advance the formation of the amorphous film and the crystallization of the metal oxide film. Practically, all of the solution strategies developed by us take advantage of the complementarity and, in some cases, synergy among the physico-chemical mechanisms induced in the film by the irradiation with UV-light and others derived from different low-temperature solution approaches described in the literature [5, 11, 14].
3 Low-temperature solution processing of crystalline films assisted by continuous UV-irradiation
We have devised successful solution approaches that have mainly made use of the assistance of continuous UV-irradiation for decreasing the processing temperature of crystalline metal oxide films [11]. This strategy makes use of photochemistry by which the interaction of light with a material is able to produce the breaking of chemical bonds and to facilitate the formation of new ones. If molecules are cleaved into smaller molecules or individual atoms, new entities are formed upon photochemical reactions, which can further assist to the elimination of organic residuals, to promote condensation and compaction of the amorphous metal oxygen network and even to accelerate the crystallization of the metal oxide [11,12,13, 15,16,17]. These UV-light irradiation can induce photochemical reactions in (a) the precursor solution, (b) the solution-deposited layer and (c) the irradiation atmosphere. Figure 3 shows schematically how these irradiation processes occur. Different strategies reported by our group for the low-temperature processing of complex metal oxides, like ferroelectric perovskites (with crystallization temperatures over 600 °C) on flexible plastic substrates (with degradation temperatures below 400 °C) are described in the next paragraphs.
Diagram showing the main steps of the Chemical Solution Deposition of crystalline thin films, schematically showing that photochemical reactions can be induced with UV-light in the precursor solution, the solution-deposited layer and the irradiation atmosphere, with the aim of advancing the crystallization of the metal oxide film
We have developed an approach based on the heterogeneous photocatalysis of precursor solutions to attain low-temperature liquid precursors [18]. These precursors have been used to fabricate crystalline BiFeO3 films at temperatures ≤350 °C (Fig. 4). In this approach, photocatalytic particles of TiO2 are added to the precursor solution and the resulting suspension is illuminated at room temperature with a light source with peak absorption maxima in the UV region. During irradiation of the solution, the metal precursors attached to the surface of the TiO2 particles undergo photocatalytic reactions, which are induced by the TiO2 particles upon light absorption. This results in the chemical breakdown of most of the organic entities of the solution, advancing their decomposition inside the solution. After this photocatalytic process and the separation of the TiO2 particles by centrifugation from the solution, a photo-catalyzed liquid precursor with a reduced content of organics is accomplished, which was successfully applied to the direct deposition of ferroelectric crystalline BiFeO3 thin films on flexible plastic substrates.
Heterogeneous photocatalysis of precursor solutions to obtain ferroelectric BiFeO3 perovskite thin films on flexible polyimide substrates. Adapted with permission from ref. [18]. Copyright 2015 Wiley-VCH Verlag GmbH & Co. KGaA
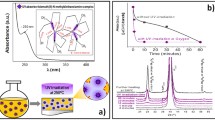
Strategies based on the synthesis of photosensitive precursor solutions have been also developed by our group [19,20,21,22]. Charge transfer excited states play an important role in the photochemistry of metal coordination complexes because metal-to-ligand charge transfer (MLCT) transitions induced by UV-light can occur. Therefore, we can take advantage of the photoreactions produced in these metal complexes as a result of MLCT transitions under illumination to prepare materials difficult to obtain otherwise, like crystalline metal oxide thin films at low temperatures. Figure 5 shows how the synthesis in solution of photosensitive molecular precursors (e.g., β-diketonate or N-methyldiethanolamine metal complexes) with a high absorption in the UV-range (Fig. 5a), makes possible the preparation of β-Bi2O3 films at a very low temperature of 250 °C and thus their direct deposition on cheap plastic substrates (Fig. 5b), obtaining efficient supported photocatalytic materials. It should be noted that the β-Bi2O3 polymorph is a metastable phase which is stable in a high-temperature interval (497–667 °C). Therefore, the solution strategy used in this work makes possible for the first time the crystallization of films of this high-temperature polymorphic form at temperatures more than 300 °C below [21]. This type of molecular precursors has been also applied to the fabrication at low temperature of complex metal oxide films, like the ferroelectric PbTiO3 films shown in Fig. 5c, which were prepared at 400 °C on Pt-coated Si substrates, showing this strategy the beneficial effect of the UV-irradiation of photosensitive precursors for the preparation of thin films with appropriate functional properties.
Photosensitive precursor solutions used for the low-temperature processing of crystalline metal oxide films. a Metal complexes synthesized in the precursor solutions showing a maximum of absorption in the UV range. b Photocatalytic films of the metastable β-Bi2O3 phase stabilized by this strategy on polyimide substrates at a processing temperature of 250 °C. c Surface microstructure of PbTiO3 thin films on Pt-coated silicon substrates prepared at 400 °C from photosensitive solutions, showing the enhanced ferroelectric response of the UV-irradiated films
To reinforce the ability of these solutions for producing crystalline metal oxide films at low temperatures, seeded photosensitive solutions have been successfully deposited on flexible polyimide for the fabrication of films of the Pb(ZrxTi1-x)O3 perovskite, a high responsive ferro-piezoelectric material, and of the multiferroic BiFeO3 perovskite [23,24,25]. These films are able to be crystallized at very low temperatures, between 300 °C and 350 °C, providing flexible ferroelectric materials with excellent functional properties (Fig. 6). In this strategy, seeds are added to the synthesized photosensitive solutions. The UV-irradiation of the photoactive films deposited from the seeded photosensitive precursors produces the prompt dissociation of the metal complexes and the decomposition of the organic ligands, with the subsequent formation and spreading of a metal–oxygen network. Meanwhile, the seeds occluded in the deposited layer increase the number of nucleation sites in the film, accelerating the crystal nucleation and its growth, thus resulting in a significant decrease in the crystallization temperature of the film.
Seeded photosensitive solutions used for the fabrication of ferroelectric Pb(ZrxTi1-x)O3 perovskite films on flexible polyimide at processing temperatures ≤350 °C. Adapted with permission from ref. [23]. Copyright 2014 Wiley-VCH Verlag GmbH & Co. KGaA
The in-situ generation of seeds in the precursor solution is also possible and for that we have developed a novel self-induced solution seeding approach (Fig. 7), which has permitted the fabrication of BiFeO3 perovskite films on flexible polyimide substrates [26]. This strategy makes use of the controlled addition of an antisolvent, 1,3-propanediol, to an acetic acid solution of Bi(III) and Fe(III) salts. This addition results in the in-situ formation of nanocrystals (seeds) by supersaturation in the precursor solution. These nanocrystalline seeds accelerate the crystallization of the perovskite phase in the solution-deposited layer, allowing the direct growth of BiFeO3 perovskite films on polymeric substrates at 350 °C. These low-temperature flexible BiFeO3 films have significant ferroelectric, photovoltaic and photocatalytic properties of interest in multiple applications.
Scheme of the self-induced solution seeding approach devised for the low-temperature fabrication of BiFeO3 perovskite thin films directly on polyimide substrates. Adapted with permission from ref. [26]. Copyright 2022 Wiley-VCH Verlag GmbH & Co. KGaA
The former strategies developed for the low-temperature fabrication of crystalline thin films are focused on the activation of the sol(solution) precursor and/or the solution-derived film, mainly with the assistance of UV-light. However, the gas atmosphere where the film is UV-irradiated can also play an important role in the processing of the film (see Fig. 3). The irradiation of gas molecules produces their photolysis, which results in the formation of reactive free radicals. This process is followed by secondary reactions such as the attack of the early radicals to the gas molecules forming new free radicals and molecular species in the atmosphere. These chemically reactive species can accelerate the formation of the intermediate amorphous metal oxide film and, therefore advance the formation of the crystalline phase. We call this strategy film crystallization induced by photochemically generated reactive radicals [11, 23, 27,28,29]. Oxygen (O2) atmospheres are the most used during the UV-irradiation of solution-deposited metal oxide thin films. This is because the photolysis of O2 produces free oxygen radicals (O•) that react with molecular O2 forming ozone (O3). O3 is a strong oxidizing agent that enhances the decomposition of the organic compounds from the film by ozonolysis, whereas the O• radicals can compensate the charge defects developed in the crystal lattice, improving the oxide stoichiometry. Atmospheres specially designed to promote specific chemical reactions can be used in this approach. This is the case of using O2 atmospheres enriched with water vapor (H2O). Here, the UV-irradiation produces O• radicals by the photolysis of O2 and these O• free radicals are able to react with H2O giving rise •OH radicals. The highly reactive •OH species produce a quick nucleophilic attack to the chemical compounds contained in the solution-derived film, enhancing the condensation of the metal oxygen network and promoting the formation of a high-densified, defect-free amorphous metal oxide film that is able to convert it into the crystalline phase by applying a low amount of energy (i.e. thermal treatments at low temperatures). By using this strategy (Fig. 8), crystalline metal oxide films have been prepared directly on plastic substrates in a low-temperature regime, between 250 °C and 350 °C, observing a gradual increase in the processing temperature as the complexity of the metal oxide increases (from binary to ternary or quaternary metal oxides: e.g., the photocatalytic Bi2O3, the multiferroic BiFeO3 or the ferro-piezoelectric Pb(ZrxTi1−x)O3) [29].
We foresee that the combination and synergy of different low-temperature solution strategies can be a powerful tool for the fabrication of crystalline metal oxide films at temperatures below those currently reported, thus fulfilling the today demands about the deposition of high-performance crystalline metal oxide thin films on cheap, lightweight and flexible substrates, using sustainable fabrication processes with low-energy consumption and reduced carbon footprint. A first and relevant attempt to achieve this target is the preparation by CSD of functional BiFeO3 thin films on plastic substrates at a lower temperature limit of 325 °C, by making use of the complementarity of different low-temperature solution strategies (Fig. 9) [30]. In a first stage, this approach synthesizes designed molecular metal complexes in solution with the aim of obtaining photosensitive precursor solutions. The molecular structure of these complexes make them photosensitive compounds besides to have a molecular structure close to that of the crystalline phase [31], which facilitates the amorphous-to-crystalline conversion. In addition, the reactions among the chemical compounds contained in these solutions (e.g., oxidizing and reducing chemical agents) result in internal film combustions [32, 33] during processing, providing an internal energy to the system that contributes to the crystallization of the film. Finally, reactive radicals [11,12,13,14] are formed in the O2 atmosphere used during the film irradiation, which produces O3 and enhances the decomposition of the organics.
Scheme showing how the complementarity among different solution deposition strategies can be exploited for the low-temperature fabrication of multifunctional BiFeO3 perovskite thin films on flexible plastic substrates. With permission from ref. [30]. Copyright 2020 Wiley-VCH Verlag GmbH & Co. KGaA
4 Summary and outlook
The preparation of functional crystalline metal oxide films at low temperatures on cheap, temperature-sensitive polymer substrates, is demanded today in the emerging Flexible Electronics. However, the crystallization of metal oxides usually requires temperatures over the degradation temperatures of such substrates (≤400 °C). This fact along with additional benefits such as a low-energy consumption and a reduced carbon footprint has pushed the development of low-temperature fabrication processes that need to be scalable to the sizes of flexible devices. This paper shows the different low-temperature solution methods developed in our group for the fabrication of crystalline metal oxide thin films. This overview was discussed in the invited talk presented at the Solgel 2022 International Conference celebrated in Lyon, France. Among the investigated approaches, the use of continuous UV-irradiation during the solution deposition of films has been exploited for attaining a quick decomposition of the organic compounds and for advancing the formation of an amorphous interconnected metal oxygen network. This amorphous metal oxide film has a high bulk density and a low content of defects, characteristics that result in a significant reduction of the energy activation barrier that needs to be surpassed for the film crystallization. As a result, a lower amount of thermal energy has to be supplied to the system for the film crystallization and functional crystalline thin films can be directly fabricated on flexible polymers at temperatures ≤350 °C. The combination of different low-temperature solution deposition strategies is foresee as a powerful tool for achieving the further reduction of the crystallization temperature of metal oxide thin films, which is requested for the integration of crystalline films with bio-based, bio-compatible or bio-degradable polymers. We are convinced of the great potential of sol(solution)-gel processing for the low-temperature fabrication of crystalline metal oxide thin films. Solution methods open novel pathways to synthesize thermodynamically disfavored chemical compounds and to overcome energy barriers otherwise not possible with other metal oxide film fabrication techniques, which is a key asset to fulfill the demands of the upcoming electronic devices.
References
Park JW, Kang BH, Kim HJ (2020) A review of low-temperature solution-processed metal oxide thin-film transistors for flexible electronics. Adv Funct Mater 30:1904632
Bretos I, Jiménez R, Ricote J, Calzada ML (2020) Low-temperature solution approaches for the potential integration of ferroelectric oxide films in flexible electronics. IEEE Trans Ultrason Ferroelectr Freq Control 6(10):1967–1979
Kozuka H (2013) Wet processing for the fabrication of ceramic thin films on plastics. J Mater Res 28:673–688
Kozuka H (2018) Sol-gel preparation of crystalline oxide thin films on plastics. In: Klein L et al. (eds.) Handbook of sol-gel science and technology. Springer International Publishing Switzerland, p. 1–21. https://doi.org/10.1007/978-3-319-19454-7_147-1.
Bretos I, Jiménez R, Ricote J, Calzada ML (2018) Low-temperature crystallization of solution derived metal oxide thin films assisted by chemical processes. Chem Soc Rev 47:291–308
Xu W, Li H, Xu JB, Wang L (2018) Recent advances of solution-processed metal oxide thin-film transistors. ACS Appl Mater Interfaces 10:25878–25901
Communication from the Commision COM 2021/118. The European Green Deal. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=COM%3A2019%3A640%3AFIN.
Communication from the Commision COM 2019/640. The European Green Deal. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=COM%3A2019%3A640%3AFIN.
Ji D, Cai S, Paudel TR, Sun H, Zhang C, Han L, Wei Y, Zang Y, Gu M, Zhang Y, Gao W, Huyan H, Guo W, Wu D, Gu Z, Tsymbal EY, Wang P, Nie Y, Pan X (2019) Freestanding crystalline oxide perovskites down to the monolayer limit. Nature 570:87–91
Calzada ML, Bretos I, Jimenez R, Guillon H, Pardo L (2004) Low-temperature processing of ferroelectric thin films compatible with silicon integrated circuit technology. Adv Mater 16(18):1620–1624
Bretos I, Jiménez J, Ricote J, Calzada ML (2020) Photochemistry in the Low-Temperature Processing of Metal Oxide Thin Films by Solution Methods. Chem Eur J 26:9277–9291
Imai H, Tominaga A, Hirashima H, Toki M, Aizawa M (1998) Ultraviolet-laser-induced crystallization of sol-gel derived indium oxide films. J Sol Gel Sci Technol 13:991–994
Imai H (2005) Ultraviolet (UV) Irradiation, In: Sakka S. (ed.), Handbook of sol-gel science and technology: processing, characterization and applications. chp. 27, vol. 1 Kluwer Academic Publishers, Dordrecht, p. 639–650.
Bretos I, Diodati S, Jimenez R, Tajoli F, Ricote J, Bragaggia G, Franca M, Calzada ML, Gross S (2020) Low-temperature solution crystallization of nanostructured oxides and thin films. Chem Eur J 26(42):157–9179.
Nakajima T, Shinoda K, Tsuchiya T (2014) UV-assisted nucleation and growth of oxide films from chemical solutions. Chem Soc Rev 43(7):2027–2041
Boyd W, Zhang JY (1997) New large area ultraviolet lamp sources and their applications. Nucl Instrum Methods Phys Res, Sect B 121(4):349–356
Tohge N, Shinmou K, Minami T (1994) Effects of UV-Irradiation on the Formation of Oxide Thin Films from Chemically Modified Metal-Alkoxides. J Sol Gel Sci Technol 2:581–585
Bretos I, Jiménez J, Pérez-Mezcua D, Salazar N, Ricote J, Calzada ML (2015) Low-temperature liquid precursors of crystalline metal oxides assisted by heterogeneous photocatalysis. Adv Mater 27(16):2608–2613
Calzada ML, Gonzalez A, Poyato R, Pardo L (2003) Photo-sensitive sol-gel solutions for the low-temperature UV-assisted processing of PbTiO3 based ferroelectric thin films. J Mater Chem 13(6):1451–1457
De Dobbelaere C, Calzada ML, Jimenez R, Ricote J, Bretos I, Mullens J, Hardy A, Van Bael MK (2011) Aqueous solutions for low-temperature photoannealing of functional oxide films: reaching the 400 °C Si-technology integration barrier. J Am Chem Soc 133(33):12922–12925
Perez-Mezcua D, Sirera R, Jimenez R, Bretos I, De Dobbelaere C, Hardy A, Van Bael MK, Calzada ML (2014) A UV-absorber bismuth(III)-N-methyldiethanolamine complex as a low-temperature precursor for bismuth-based oxide thin films. J Mater Chem C 2(41):8750–8760
Bretos I, Jimenez R, Garcia-Lopez J, Pardo L, Calzada ML (2008) Photochemical solution deposition of lead-based ferroelectric films: avoiding the PbO-excess addition at last. Chem Mater 20(18):5731–5733
Bretos I, Jimenez R, Wu AY, Kingon AI, Vilarinho PM, Calzada ML (2014) Activated solutions enabling low-temperature processing of functional ferroelectric oxides for flexible electronics. Adv Mater 26(9):1405–1409
Bretos I, Jimenez R, Tomczyk M, Rodriguez-Castellon E, Vilarinho PM, Calzada ML (2016) Active layers of high-performance lead zirconate titanate at temperatures compatible with silicon nano- and microelecronic devices. Sci Rep. 6:20143
Tomczyk M, Bretos I, Jimenez R, Mahajan A, Ramana EV, Calzada ML, Vilarinho PM (2017) Direct fabrication of BiFeO3 thin films on polyimide substrates for flexible electronics. J Mater Chem C 5(47):12529–12537
Barrios O, Jimenez R, Ricote J, Tartaj P, Calzada ML, Bretos I (2022) A sustainable self-induced solution seeding approach for multipurpose bifeo3 active layers in flexible electronic devices. Adv Funct Mater 32(7):2112944
Park S, Kim KH, Jo JW, Sung S, Kim KT, Lee WJ, Kim J, Kim HJ, Yi GR, Kim YH, Yoon MH, Park SK (2015) In-depth studies on rapid photochemical activation of various sol–gel metal oxide films for flexible transparent electronics. Adv Funct Mater 25:2807–2815
Cochran EA, Woods KN, Johnson DW, Page CJ, Boettcher SW (2019) Unique chemistries of metal-nitrate precursors to form metal-oxide thin films from solution: materials for electronic and energy applications. J Mater Chem A 7:24124
Gomez-Lopez A, Rivas YA, Lopez-Fajardo S, Jimenez R, Ricote J, Pecharroman C, Montero I, Bretos I, Calzada ML (2023) In situ photogenerated hydroxyl radicals in the reaction atmosphere for the accelerated crystallization of solution-processed functional metal oxide thin films. J Mater Chem C. https://doi.org/10.1039/d2tc05447g.
Bretos I, Jimenez R, Ricote J, Sirera R, Calzada ML (2020) Photoferroelectric thin films for flexible systems by a three-in-one solution-based approach. Adv Funct Mater 30(32):2001897
Perez-Mezcua D, Bretos I, Jimenez R, Ricote J, Jimenez-Rioboo RJ, da Silva CG, Chateigner D, Fuentes-Cobas L, Sirera R, Calzada ML (2017) Photochemical solution deposition of beta-Bi2O3 thin films. J Sol Gel Sci Technol 81(2):355–361
Kim MG, Kanatzidis MG, Mercouri G, Facchetti A, Marks TJ (2011) Low-temperature fabrication of high-performance metal oxide thin-film electronics via combustion processing. Nat Mater 10(5):382–388
Pujar P, Gandla S, Gupta D, Kim S, Kim MG (2020) Trends in low-temperature combustion derived thin films for solution-processed electronics. Adv Elect Mater 6:2000464
Funding
This work was supported by Spanish Projects PID2019-104732RB-I00 and TED2021-130871B-C21 funded by MCIN/AEI/10.13039/501100011033. ME acknowledges the financial support of the JAE Intro 2022 ICU ICMM. Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bretos, I., Jiménez, R., Ricote, J. et al. Low-temperature sol–gel methods for the integration of crystalline metal oxide thin films in flexible electronics. J Sol-Gel Sci Technol 107, 269–277 (2023). https://doi.org/10.1007/s10971-023-06065-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-023-06065-2