Abstract
Porous MgF2 antireflective λ/4 films were prepared by sol–gel processing and coated with an additional top layer by electron beam evaporation. Scanning Electron Microscopy was applied to characterize the microstructure of the bilayer assembly. It can be shown that the top layer has a protective effect in terms of abrasion resistance and reduced solubility in water. In a second step the thickness of the two film systems has been matched to achieve optimum antireflection properties.

Porous antireflective MgF2 coatings were prepared by sol–gel processing. On these samples dense MgF2 films were deposited by evaporation in order to increase the stability of the system.
Highlights
-
MgF2 was evaporated on sol–gel derived porous antireflective MgF2 coatings.
-
The microstructure of the bilayer assemblies was characterized by scanning electron microscopy (SEM).
-
The evaporated layer provides improved abrasion resistance and reduced water solubility.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Sol–gel processing has turned out to be a viable tool for the preparation of λ/4 antireflective coatings [1]. In contrast to the application of interference filters, only a single film is required. However, in order to obtain the intermediate position between glass substrates and ambient atmosphere λ/4 films have to contain some porosity. In the case of SiO2 a porosity of 50% is needed which entails restrictions in terms of mechanical stability. As MgF2 has a lower refractive index than SiO2, such antireflective films only require a porosity of 30% which goes along with an enhanced abrasion resistance [2].
Very promising results were achieved for MgF2 antireflective coatings [3, 4] and such systems even passed the harsh steady-state temperature humidity life test where samples are exposed to 85% relative humidity at 85 °C (85/85) [5]. Nevertheless, such systems exhibited a surprising and unpleasant solubility in water at room temperature [6]. In this paper we describe how porous MgF2 films can be protected by an additional top coat prepared by e-beam evaporation.
2 Experimental procedure
2.1 Synthesis of coating solutions and film preparation
Dried methanol was purchased from Carl Roth (max. 50 ppm H2O). Magnesium ethoxide Mg(OEt)2 was obtained from STREM Chemicals (97%). MgCl2 was obtained from Sigma Aldrich.
The synthesis of the MgF2 coating solutions is based on a method previously reported [7]: MgCl2 was dissolved in methanol before Mg(OEt)2 was added (molar ratio 15:85, overall Mg concentration 0.6 M) The Mg(OEt)2 did initially did not dissolve. The dispersion was stirred for 10 min before methanolic HF (23.3 M) is added slowly under vigorous stirring. The reaction mixture is stirred for at least one day and resulted in a clear solution.
MgF2 thin films were prepared by dip coating on borosilicate glass (Schott Borofloat®) at the size of 3.3 * 150 * 100 mm. Before the coating experiment, the substrates were cleaned in a laboratory dishwasher by an alkaline cleaning procedure with a final neutralization step. Final thermal curing of films was performed by placing the samples in an oven (Model Thermicon P, Heraeus Instruments, Hanau, Germany) at ambient conditions. Then the temperature was raised to 500 °C within two hours. After a dwell time of 10 min the samples were allowed to cool down to room temperature overnight.
Additional MgF2 was deposited on the sol–gel derived films by e-beam evaporation. This procedure was carried on a Balzers BAK 760 box coater with a nearly cubic vacuum chamber of about (0,9 m)3. The process parameters were as usual for optical coatings on mineral substrates: a starting pressure of 2*10-5 mbar, a temperature of 275 °C, an evaporation rate of 1.2 nm/s and a thickness of 40 nm.
2.2 Material characterization
The transmittance and reflectance of films in the spectral range between 300 nm and 2400 nm was determined using a Shimadzu UV-3100 UV-VIS-NIR recording spectrometer combined with a MPC-3100 multi-purpose large sample compartment for UV-3100. The respective film thickness was calculated according the method described by Diaz [8].
Scanning electron microscopy (SEM) images were taken using a Ultra 55 (Carl Zeiss NTS GmbH, Germany) scanning electron microscope. Specimen were prepared by mechanically fracturing and evaporation of a thin (1–2 nm) conductive layer of carbon.
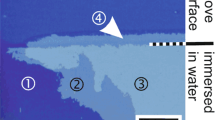
The mechanical stability of the films was tested by a custom made crockmeter test using steel wool of the fineness 0000 as abrasive. The stamp (contact area 4.5 cm²) was pressed on the sample with a force of 4 N. For testing the film solubility samples were partially immersed into an excess of stirred distilled water at room temperature and visually monitored as a function of time.
3 Results and discussion
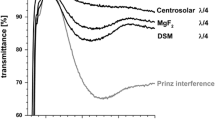
Sol–gel processing by dip-coating results in material deposition on both sides of a substrate. By choosing the withdrawal rate the film thickness of porous MgF2 can be adjusted to prepare so-called λ/4 layers that provide a minimum of reflectance at a wavelength λ. In Fig. 1 the spectrum of such a sample with a minimum reflectance in the visual range is given. In contrast to that evaporation techniques only yield a single side deposition. In a first step such a single MgF2 coating with a thickness of 40 nm was prepared on one side of a porous double sided MgF2 system in order to evaluate the structural compatibility of the two techniques. As can be seen from Fig. 1 this procedure leads to a slight increase of reflectance and a shift of the spectral minimum to higher wavelengths. The refractive index (550 nm) of the porous MgF2 and the evaporated MgF2 were found to be 1275 and 1384 respectively.
Scanning electron microscopy (SEM) was applied to characterize the different deposits, in Fig. 2 the respective top views are given. The MgF2 prepared by sol–gel processing exhibits an open porous granular structure. In areas where MgF2 is deposited directly on glass by electron beam evaporation a significantly finer surface pattern is observed and there is no indication of pores within the film.
When MgF2 is evaporated on top of a porous sol–gel derived film the granular structure of the underlying material remains visible, but the pore size is reduced. It seems as if the evaporation results in a structurally conformal deposition of MgF2.
Cross-sectional views of the above sample regions also were prepared (Fig. 3). The sol–gel derived MgF2 film reveals a porous microstructure. The MgF2 grains appear coarser than the top-view suggested. The direct electron beam evaporation on glass results in a columnar growth which is commonly reported for this method [9]. These structural features also can be observed when MgF2 is evaporated on the porous sol–gel film, no clear demarcation appears in SEM imaging.
The thickness of the porous film (125 nm) has been evaluated by UV-Vis spectroscopy independent from SEM. Film growth (40 nm) during e-beam evaporation was monitored by a microbalance. These two values are more reliable than the results that are taken from SEM imaging in Fig. 3, because errors may result e.g. from the tilt of the specimen.
The MgF2 evaporation on top of the porous sol–gel coating had the aim of increasing its mechanical stability and strength against dissolution in water. In Fig. 4 the results of crockmeter testing are given. The porous sol–gel derived coating alone already shows a good result, only minor abrasion can be seen. One has to keep in mind that steel wool instead of felt is used as the abrasive.
The sample with an additional 40 nm MgF2 deposited by evaporation shows comparable results. After 50 cycles the surface appears in a deeper blue (better antireflective properties) than the unimpacted film. This indicates that the 40 nm top coating is partially damaged and the underlying porous sol–gel film with its reflectance minimum in the visual range emerges at the surface.
In Fig. 5 the results from water solubility testing are compared. Whereas the porous file undergoes significant damage after exposure to water for six days, the samples with a dense 40 nm topcoat remain undamaged.
The above investigations have been made using porous MgF2 coating derived from sol–gel processing. Their thickness of 125 nm is chosen for best antireflective properties in air, their optical performance is worsened by the additional dense 40 nm MgF2 (Fig. 1). This top layer turned out to improve the stability of the underlying porous film. Therefore, the film thickness of both systems has to be adjusted to each other in order to minimize the reflectivity of the complete film stack.
The antireflective properties of evaporated MgF2 on a sol–gel MgF2 layer were modeled using the commercial Software TFCalc (v3.5.15, Software Spectra Inc.). The substrate was borofloat glass (3 mm) and the stack of layers was sol–gel MgF2 directly on the substrate and evaporated MgF2 on top of the sol–gel layer. The required n-k-data was taken from the database of the modeling software for the substrate and evaporated MgF2 whereas the dielectric properties of sol–gel MgF2 were derived from ellipsometric measurements of the particular layers used. The thickness of the sol–gel MgF2 was adjusted towards minimal reflection at 550 nm. It could be observed that the wavelength of the reflection minimum increases with increasing layer thickness, while the total remaining reflection of the minimum decreases. This is in good agreement with the theory of λ/4 coatings when assuming the stack of evaporated MgF2 and sol–gel MgF2 as an effective medium. 40 nm evaporated MgF2 require 57 nm sol–gel layer thickness to achieve 1.65% residual reflection.
Porous films with an approximate thickness of 60 nm were deposited on a borosilicate glass substrate by dip-coating, before 40 nm MgF2 was evaporated on both sides of these samples. The reflectance spectrum of such a sample is given in Fig. 6. The degree of minimum residual reflectance of 1.50% is in good agreement with the value theoretically calculated (1.65%).
4 Conclusions
It is possible to stabilize sol–gel derived MgF2 antireflective coatings by means of an additional MgF2 top layer deposited by electron beam evaporation. The optical performance of such multilayer assemblies can be optimized by adjusting both layer thicknesses.
References
Löbmann P (2013) Antireflective coatings and optical filters. In: Schneller T, Waser R, Kosec M, et al. (eds.) Chemical solution deposition of functional oxide thin films, vol 29. Springer Vienna, Vienna, pp 707–724.
Löbmann P (2017) Antireflective coatings by sol–gel processing: commercial products and future perspectives. J Sol-Gel Sci Technol 83:291–295. https://doi.org/10.1007/s10971-017-4408-x
Noack J, Scheurell K, Kemnitz E et al. (2012) MgF2 antireflective coatings by sol–gel processing: film preparation and thermal densification. J Mater Chem 22:18535. https://doi.org/10.1039/c2jm33324d
Löbmann P (2018) Sol-Gel processing of MgF2 antireflective coatings. Nanomaterials 8. https://doi.org/10.3390/nano8050295
Scheurell K, Kemnitz E, Garcia-Juan P et al. (2015) Porous MgF2 antireflective λ/4 films prepared by sol–gel processing: comparison of synthesis approaches. J Sol-Gel Sci Technol 76:82–89. https://doi.org/10.1007/s10971-015-3754-9
Hegmann J, Jahn R, Löbmann P (2017) Solubility of porous MgF2 films in water: influence of glass substrates. J Sol-Gel Sci Technol 82:40–44. https://doi.org/10.1007/s10971-016-4280-0
Krahl T, Broßke D, Scheurell K et al. (2016) Novel aspects in the chemistry of the non-aqueous fluorolytic sol–gel synthesis of nanoscaled homodisperse MgF2 sols for antireflective coatings. J Mater Chem C 4:1454–1466
Dıaz-Parralejo A, Caruso R, Ortiz AL et al. (2004) Densification and porosity evaluation of ZrO2–3 mol.% Y2O3 sol–gel thin films. Thin Solid Films 458:92–97
Schmidl L, Schmidl G, Gawlik A et al. (2019) Combining super-resolution microscopy with neuronal network recording using magnesium fluoride thin films as cover layer for multi-electrode array technology. Sci Rep. 9:16110. https://doi.org/10.1038/s41598-019-52397-x
Acknowledgements
The authors thank the Kemnitz group (Humboldt University Berlin) for the synthesis of the MgF2 coating solution. This project was funded by the German Federal Ministry of Economics and Technology (grant 03EN1019A).
Funding
This work was supported by Gerd-Peter Scherg, Rodenstock GmbH in Munich, Germany. Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Steenhusen, S., Scherg, GP. & Löbmann, P. Increased durability of sol–gel derived MgF2 antireflective coatings capped by vapor deposition. J Sol-Gel Sci Technol 105, 58–62 (2023). https://doi.org/10.1007/s10971-022-05977-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-022-05977-9