Abstract
The purpose of this research is to develop a hydrogel delivery system for solubility enhancement and sustained release of hydrophobic drugs. The model drug, diflunisal, has documented poor solubility in water which limits its application and administration. In this research, polyamidoamine (PAMAM) dendrimer is employed to dramatically increase the water solubility of diflunisal. In the meantime, PAMAM dendrimer functionalized with crosslinkers is further in situ crosslinked with thiolated polyethylene glycol (PEG) to form a hydrogel, encapsulating a high payload of diflunisal for sustained release. The diflunisal encapsulated hydrogel is immersed in water or laid on top of mouse skin, and the amount of diflunisal released from the gel is quantitatively measured by UV-Vis spectroscopy. Results show that the solubility of diflunisal in water is improved by 129–193 folds in the presence of PAMAM dendrimer at the experimental conditions compared to the reported solubility, and the amount of diflunisal is released by up to 87% of its load and reaches its maximum amounts after 72 h. This research demonstrates that the PAMAM dendrimer-PEG hydrogel system may serve as a promising delivery system for hydrophobic drugs.

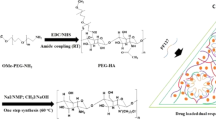
Vinyl sulfone functionalized PAMAM dendrimer increases the water solubilty of hydrophobic anti-inflammatory drug diflunisal, and crosslinks with thiolated mutibranched PEG to form a hydrogel. A large payload of diflunisal is released from hydrogel and penetrates into mouse skin in a controllable manner depending on the hydrogel crosslinking density which can be chemically modified.
Highlights
-
PAMAM dendrimer-mediated hydrogel platforms with tunable structures for sustained drug delivery.
-
129–193-fold solubility increase of hydrophobic drug diflunisal.
-
74–87% drug release in 72 h either in aqueous media or through mouse skin penetration.






Similar content being viewed by others
References
Narayanaswamy R, Torchilin VP (2019) Hydrogels and their applications in targeted drug delivery. Molecules 24(3):603. https://doi.org/10.3390/molecules24030603
He C, Ji HF, Qian YH, Wang Q, Liu XL, Zhao WF, Zhao CS (2019) Heparin-based and heparin-inspired hydrogels: size-effect, gelation and biomedical applications. J Mater Chem B 7:1186–1208. https://doi.org/10.1039/C8TB02671H
Wei W, Zhang Q, Zhou WY, Liu ZM, Wang YF, Alakpa EV, Ouyang HW, Liu H (2019) Immunomodulatory application of engineered hydrogels in regenerative medicine. Appl Mater Today 14:126–136. https://doi.org/10.1016/j.apmt.2018.11.013
Wang X, Chen SS, Wu DB, Wu Q, Wei QC, He B, Lu QH, Wang QG (2018) Oxidoreductase-initiated radical polymerizations to design hydrogels and micro/nanogels: mechanism, molding, and applications. Adv Mater 30(17):1705668. https://doi.org/10.1002/adma.201705668
Anjana J, Mohandas A, Seethalakshmy S, Suresh MK, Menon R, Biswas R, Jayakumar R (2018) Bi-layered nanocomposite bandages for controlling microbial infections and overproduction of matrix metalloproteinase activity. Int J Biol Macromolecules 110:124–132. https://doi.org/10.1016/j.ijbiomac.2017.12.043
Desai PN, Yuan Q, Yang H (2010) Synthesis and characterization of photocurable polyamidoamine dendrimer hydrogels as a versatile platform for tissue engineering and drug delivery. Biomacromolecules 11(3):666–673. https://doi.org/10.1021/bm901240g
Kim SH, An YH, Kim HD, Kim K, Lee SH, Yim HG, Kim BG, Hwang NS (2018) Enzyme-mediated tissue adhesive hydrogels for meniscus repair. Int J Biol Macromolecules 110:479–487. https://doi.org/10.1016/j.ijbiomac.2017.12.053
Iemma F, Spizzirri UG, Puoci F, Muzzalupo R, Trombino S, Cassano R, Leta S, Picci N (2006) pH-Sensitive hydrogels based on bovine serum albumin for oral drug delivery. Int J Pharmaceutics 312(1-2):151–157. https://doi.org/10.1016/j.ijpharm.2006.01.010
Yang JA, Yeom J, Hwang BW, Hoffman AS, Hahn SK (2014) In situ-forming injectable hydrogels for regenerative medicine. Prog Polym Sci 39(12):1973–1986. https://doi.org/10.1016/j.progpolymsci.2014.07.006
Park KM, Park KD (2018) In situ cross-linkable hydrogels as a dynamic matrix for tissue regenerative medicine. Tissue Eng Regen Med 15(5):547–557. https://doi.org/10.1007/s13770-018-0155-5
Zheng YT, Wang WF, Zhao JL, Wu CY, Ye CQ, Huang MX, Wang SG (2019) Preparation of injectable temperature-sensitive chitosan-based hydrogel for combined hyperthermia and chemotherapy of colon cancer. Carbohydr Polym 222:115039. https://doi.org/10.1016/j.carbpol.2019.115039
Liu CJ, Ruan CP, Shi R, Jiang BP, Ji SC, Shen XC (2019) A near infrared-modulated thermosensitive hydrogel for stabilization of indocyanine green and combinatorial anticancer phototherapy. Biomater Sci 7(4):1705–1715. https://doi.org/10.1039/c8bm01541d
Lei KW, Tang L (2019) Surgery-free injectable macroscale biomaterials for local cancer immunotherapy. Biomater Sci 7(3):733–749. https://doi.org/10.1039/c8bm01470a
Yegappan R, Selvaprithiviraj V, Amirthalingam S, Jayakumar R (2018) Carrageenan based hydrogels for drug delivery, tissue engineering and wound healing. Carbohydr Polym 198:385–400. https://doi.org/10.1016/j.carbpol.2018.06.086
Staruch RMT, Glass GE, Rickard R, Hettiaratchy SP, Butler PEM (2017) Injectable pore-forming hydrogel scaffolds for complex wound tissue engineering: designing and controlling their porosity and mechanical properties. Tissue Eng Part B-Rev 23(2):183–198. https://doi.org/10.1089/ten.teb.2016.0305
Winnicka K, Wroblewska M, Wieczorek P, Sacha PT, Tryniszewska E (2012) Hydrogel of Ketoconazole and PAMAM dendrimers: formulation and antifungal activity. Molecules 17(4):4612–4624. https://doi.org/10.3390/molecules17044612
Sharma A, Taniguchi J (2017) Review: Emerging strategies for antimicrobial drug delivery to the ocular surface: Implications for infectious keratitis. Ocul Surf 15(4):670–679. https://doi.org/10.1016/j.jtos.2017.06.001
Bi XD, Luckanagul JA, Allen A, Ramaboli M, Campbell E, West D, Maturavongsadit P, Brummett K, Wang Q (2015) Synthesis of PAMAM dendrimer-based fast cross-linking hydrogel for biofabrication. J Biomater Sci Polym Ed 26(11):669–682. https://doi.org/10.1080/09205063.2015.1056716
Bi XD, Liang AY, Tan Y, Maturavongsadit P, Higginbothem A, Gado TA, Gramling A, Bahn H, Wang Q (2016) Thiol-ene crosslinking polyamidoamine dendrimer-hyaluronic acid hydrogel system for biomedical applications. J Biomater Sci Polym Ed 27(8):743–757. https://doi.org/10.1080/09205063.2016.1159473
Tomalia DA, Naylor AM, Goddard WA (1990) Starburst dendrimers: molecular-level control of size, shape, surface chemistry, topology, and flexibility from atoms to macroscopic matter. Angew Chem Int Ed Engl 29:138–175. https://doi.org/10.1002/anie.199001381
Esfand R, Tomalia DA (2001) Poly(amidoamine) (PAMAM) dendrimers: from biomimicry to drug delivery and biomedical applications. Drug Discov Today 6(8):427–436. https://doi.org/10.1016/S1359-6446(01)01757-3
Yang LN, Wang HL, Li DY, Li L, Lou XF, Liu HL (2018) Self-nucleation and self-assembly of highly fluorescent Au-5 nanoclusters for bioimaging. Chem Mater 30(15):5507–5515. https://doi.org/10.1021/acs.chemmater.8b02770
Xing Y, Zhu JY, Zhao LZ, Xiong ZJ, Li YJ, Wu S, Chand G, Shi XY, Zhao JH (2018) SPECT/CT imaging of chemotherapy-induced tumor apoptosis using Tc-99m-labeled dendrimer-entrapped gold nanoparticles. Drug Deliv 25(1):1384–1393. https://doi.org/10.1080/10717544.2018.1474968
Lesniak WG, Boinapally S, Banerjee SR, Azad BB, Foss CA, Shen CT, Lisok A, Wharram B, Nimmagadda S, Pomper MG (2019) Evaluation of PSMA-targeted PAMAM dendrimer nanoparticles in a murine model of prostate cancer. Mol Pharmaceutics 16(6):2590–2604. https://doi.org/10.1021/acs.molpharmaceut.9b00181
Liu CL, Gao HQ, Zhao ZJ, Rostami I, Wang C, Zhu L, Yang YL (2019) Improved tumor targeting and penetration by a dual-functional poly(amidoamine) dendrimer for the therapy of triple-negative breast cancer. J Mater Chem B 7(23):3724–3736. https://doi.org/10.1039/c9tb00433e
Mehta P, Kadam S, Pawar A, Bothiraja C (2019) Dendrimers for pulmonary delivery: current perspectives and future challenges. N. J Chem 43(22):8396–8409. https://doi.org/10.1039/c9nj01591d
Yellepeddi VK, Kumar A, Palakurthi S (2009) Surface modified poly(amido)amine dendrimers as diverse nanomolecules for biomedical applications. Expert Opin Drug Deliv 6(8):835–850. https://doi.org/10.1517/17425240903061251
de Araujo RV, Santos SD, Ferreira EI, Giarolla J (2018) New advances in general biomedical applications of PAMAM dendrimers. Molecules 23(11):2849. https://doi.org/10.3390/molecules23112849
Choudhary S, Gupta L, Rani S, Dave K, Gupta U (2017) Impact of dendrimers on solubility of hydrophobic drug molecules. Front Pharmacol 8:261. https://doi.org/10.3389/fphar.2017.00261
Luong D, Kesharwani P, Killinger BA, Moszczynska A, Sarkar FH, Padhye S, Rishi AK, Iyer AK (2016) Solubility enhancement and targeted delivery of a potent anticancer flavonoid analogue to cancer cells using ligand decorated dendrimer nano-architectures. J Colloid Interface Sci 484:33–43. https://doi.org/10.1016/j.jcis.2016.08.061
Cheng YY, Xu TW (2005) Dendrimers as potential drug carriers. Part I. Solubilization of non-steroidal anti-inflammatory drugs in the presence of polyamidoamine dendrimers. Eur J Med Chem 40(11):1188–1192. https://doi.org/10.1016/j.ejmech.2005.06.010
Coimbra P, Fernandes D, Gil MH, de Sousa HC (2008) Solubility of diflunisal in supercritical carbon dioxide. J Chem Eng Data 53(8):1990–1995. https://doi.org/10.1021/je800384h
Giammona G, Pitarresi G, Tomarchio V, Cacciaguerra S, Govoni P (1997) A hydrogel based on a polyaspartamide: Characterization and evaluation of in-vivo biocompatibility and drug release in the rat. J Pharm Pharmacol 49(11):1051–1056. https://doi.org/10.1111/j.2042-7158.1997.tb06040.x
National Center for Biotechnology Information, (2004) U.S. National Library of Medicine, [PubChem CID:3059], https://pubchem.ncbi.nlm.nih.gov/compound/Diflunisal. Accessed 27 Apr 2022
Metabolomics Innovation Center, (2014) The Toxin and Toxin Target Database [T3D2918], http://www.t3db.ca/toxins/T3D2918. Accessed 27 Apr. 2022
Majoros IJ, Keszler B, Woehler S, Bull T, Baker JR (2003) Acetylation of poly(amidoamine) dendrimers. Macromolecules 36(15):5526–5529. https://doi.org/10.1021/ma021540e
Purpora R, Massad W, Ferrari G, Reynoso E, Criado S, Miskoski S, Pajares A, Garcia NA (2013) The NSAIDs indomethacin and diflunisal as scavengers of photogenerated reactive oxygen species. Photochemistry Photobiol 89(6):1463–1470. https://doi.org/10.1111/php.12114
Acknowledgements
The authors thank Miss Lily Song from Medical University of South Carolina who kindly provided the hairless mouse skin for our experiment of diflunisal release through skin penetration.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kegley, Z., Makay, M., Rogers, J. et al. Polyamidoamine dendrimer-polyethylene glycol hydrogel for solubility enhancement and sustained release of diflunisal. J Sol-Gel Sci Technol 104, 160–168 (2022). https://doi.org/10.1007/s10971-022-05904-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-022-05904-y