Abstract
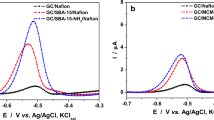
Mesoporous SBA-15-type silica, with cylindrical pores hexagonally ordered, was obtained and modified with niobium oxide in a highly dispersed way. Subsequently, a further modification with cobalt hematoporphyrin was performed. The ordered pore structure of the SBA-15 was preserved after successive modifications, maintaining its textural properties. The material was used to modify a carbon paste electrode that was successfully applied to the individual and simultaneous detection of both oxalic acid and uric acid, by using cyclic voltammetry and differential pulse voltammetry. For individual evaluation, the obtained detection limits for oxalic acid and uric acid were 9.94 and 0.17 μmol∙L−1, respectively. However, for the simultaneous evaluation of both analytes, the detection limits were 2.83 and 0.14 μmol∙L−1, for oxalic acid and uric acid, respectively.

Highlights
-
Well-ordered silica grafted with niobia and cobalt hematoporphyrin.
-
SBA-15 texture preserved after successive modifications with niobium oxide and hematoporphyrin macromolecule.
-
Simultaneous electrochemical evaluation of oxalic acid and uric acid.
-
Modified carbon paste electrode for sensitive evaluation of uric and oxalic acids.











Similar content being viewed by others
References
Zhao D, Feng J, Huo Q, Melosh N, Fredrickson G, Chmelka B, Stucky G (1998) Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 279:548–552
Galarneau A, Cambon H, Renzo FD, Ryoo R, Choi M, Fajula F (2003) Microporosity and connections between pores in SBA-15 mesostructured silicas as a function of the temperature of synthesis. N J Chem 27:73–79
Gibson LT (2014) Mesosilica materials and organic pollutant adsorption: part A removal from air. Chem Soc Rev 43:5163–5172
Didó CA, Caneppele CDG, Schneid AC, Pereira MB, Costa TMH, Benvenutti EV (2018) Small gold nanoparticles with narrow size distribution achieved in SBA-15 pores by using ionic silsesquioxane instead of thiol group as stabilizer and adhesion agente. Micropor Mesopor Mater 270:48–56
Didó CA, Mass EB, Pereira MB, Hinrichs R, D’Oca MGM, Costa TMH, Russowsky D, Benvenutti EV (2020) Heterogeneous gold nanocatalyst applied in the synthesis of 2-aryl-2,3-dihydroquinazolin-4(1H)-ones. Colloids Surf A 589:124455
AbouAitah K, Lojkowski W (2021) Delivery of natural agents by means of mesoporous silica nanospheres as a promising anticancer strategy. Pharmaceutics 13:143
Tkachenko OS, Souza LV, Deon M, Becker EM, de Menezes EW, Arenas LT, Benvenutti EV (2021) AgNP-decorated SBA-15 for MWCNT paste modified electrode: a sensor for simultaneous voltammetric determination of paracetamol and sulfamethoxazole. Electroanalysis 33:29–37
de Souza LV, da Rosa DS, Tkachenko OS, Gomes AA, Costa TMH, Arenas LT, Benvenutti EV (2019) The role silica pore structure plays in the performance of modified carbon paste electrodes. Ionics 25:3259–3268
Pessoa CA, Gushikem Y, Kubota LT (2001) Ferrocenecarboxylic acid adsorbed on Nb2O5 film grafted on a SiO2 surface: NADH oxidation study. Electrochim Acta 46:2499–2505
de Souza LV, Tkachenko O, Cardoso BN, Pizzolato TM, Dias SLP, Vasconcellos MAZ, Arenas LT, Costa TMH, Moro CC, Benvenutti EV (2019) Strategy to control the amount of titania dispersed on SBA-15 surface preserving its porosity, aiming to develop a sensor for electrochemical evaluation of antibiotics. Micropor Mesopor Mater 287:203–210
Kondo JN, Hiyoshi Y, Osuga R, Ishikawa A, Wang Y-H, Yokoi T (2018) Thin (single–triple) niobium oxide layers on mesoporous silica substrate. Micropor Mesopor Mater 262:191–198
Umpierres CS, Prola LDT, Adebayo MA, Lima EC, dos Reis GS, Kunzler DDF, Dotto GL, Arenas LT, Benvenutti EV (2017) Mesoporous Nb2O5/SiO2 material obtained by sol–gel method and applied as adsorbent of crystal violet dye. Environm Technol 38:566–578
Sumiya S, Oumi Y, Sadakane M, Sano T (2009) Facile preparation of SBA-15-supported niobic acid (Nb2O5·nH2O) catalyst and its catalytic activity. Appl Catal A 365:261–267
Arenas LT, Villis PCM, Arguello J, Landers, Benvenutti EV, Gushikem Y (2010) Niobium oxide dispersed on a carbon ceramic matrix, SiO2/C/Nb2O5, used as an electrochemical ascorbic acid sensor. Talanta 83:241–248
Xu X, Tian B, Zhang S, Kong J, Zhao D, Liu B (2004) Electrochemistry and biosensing reactivity of heme proteins adsorbed on the structure-tailored mesoporous Nb2O5 matrix. Anal Chim Acta 519:31–38
Chen Z, Prosperi M, Bird VY (2019) Prevalence of kidney stones in the USA: the National Health and Nutrition Evaluation Survey. J Clin. Urology 12:296–302
Zhang D, Li S, Zhang Z, Li N, Yuan X, Jia Z, Yang J (2021) Urinary stone composition analysis and clinical characterization of 1520 patients in central China. Sci Rep 11:6467
Dong X (2017) Study on detection methods for uric acid in biological samples. Int J Pharm Sci Res 8:926–929
Maiuolo J, Oppedisano F, Gratteri S, Muscoli C, Mollace V (2016) Regulation of uric acid metabolism and excretion. Int J Cardiol 213:8–14
Ensafi AA, Kazemzadeh A (2000) Flow injection spectrophotometric determination of ultra trace amounts of oxalic acid. Anal Chem 367:590–592
Khajehsharifi H, Pourbasheer E, Tavallali H, Sarvi S, Sadeghi M (2017) The comparison of partial least squares and principal component regression in simultaneous spectrophotometric determination of ascorbic acid, dopamine and uric acid in real samples. Arab J Chem 10:S3451–S3458
Wu FW, He ZK, Luo QY, Zeng YE (1999) HPLC determination of oxalic acid using tris(1,10-phenanthroline)ruthenium(II) chemiluminescence-application to the analysis of spinach. Food Chem 65:543–546
Li XL, Li G, Jiang YZ, Kang D, Jin CH, Shi Q, Jin T, Inoue K, Todoroki K, Toyo'oka T, Min JZ (2015) Human nails metabolite analysis: A rapid and simple method for quantification of uric acid in human fingernail by high-performance liquid chromatography with UV-detection. J Chromatogr B 1002:394–398
Zahavy E, Willner I (1996) Photoinduced electron transfer in eosin-modified Co(II)-protoporphyrin IX reconstituted myoglobin and α- or β-hemoglobin subunits: photocatalytic transformations by the reconstituted photoenzymes. J Am Chem Soc 118:12499–12514
Webb PA, Orr C, Camp RW, Olivier JP, Yunes YS (1997) Analytical methods in fine particle technology. Micromeritics Instrument Corporation, Norcross
Deon M, Caldas EM, Rosa DS, de Menezes EW, Dias SLP, Pereira MB, Costa TMH, Arenas LT, Benvenutti EV (2015) Mesoporous silica xerogel modified with bridged ionic silsesquioxane used to immobilize copper tetrasulfonated phthalocyanine applied to electrochemical determination of dopamine. J Solid State Electrochem 19:2095–2105
Pessoa CA, Gushikem Y (2001) Cobalt porphyrins immobilized on niobium (V) oxide grafted on a silica gel surface: study of the catalytic reduction of dissolved dioxygen. J Porphyr Phthalocya 5:537–544
Ribeiro ES, Dias SLP, Gushikem Y, Kubota LT (2004) Cobalt (II) porphyrin complex immobilized on the binary oxide SiO2/Sb2O3: electrochemical properties and dissolved oxygen reduction study. Electrochim Acta 49:829–834
Pottier RH, Kennedy JC, Chow YFA (1988) The pKa values of hematoporphyrin-IX as determined by absorbance and fluorescence spectroscopy. Can J Spectr 33:57–62
Lucho AMS, Oliveira EC, Pastore HO, Gushikem Y (2004) 3-n-Propylpyridinium chloride silsesquioxane polymer film-coated aluminumphosphate and adsorption of cobalt(II)tetrasulphophthalocyanine: an electrocatalytic oxidation study of oxalic acid. J Electroanal Chem 573:55–60
Chollier MJ, Epron F, Lamy-Pitara E, Barbier J (1999) Catalytic oxidation of maleic and oxalic acids under potential control of platinum catalysts. Catal Today 48:291–300
Zhou Y, Tang W, Wang J, Zhang G, Chai S, Zhang L, Liu T (2014) Selective determination of dopamine and uric acid using electrochemical sensor based on poly(alizarin yellow R) film-modified electrode. Anal Methods 6:3474–3481
Brett AMO, Brett CMA (1996) Eletroquímica Princípios, Métodos e aplicações. Almeida, Coimbra
Currie LA (1995) Nomenclature in evaluation of analytical methods including detection and quantification capabilities (IUPAC Recommendations 1995). Pure Appl Chem 67:1699–1723
Fakhari AR, Rafiee B, Ahmar H, Bagheri A (2012) Electrocatalytic determination of oxalic acid by TiO2 nanoparticles/multiwalled carbon nanotubes modified electrode. Anal Methods 4:3314–3319
Shang L, Zhao F, Zeng B (2013) Electrodeposition of PdAu alloy nanoparticles on ionic liquid functionalized graphene film for the voltammetric determination of oxalic acid. Electroanalysis 25:453–459
Raoof JB, Chekin F, Ehsani V (2015) Palladium-doped mesoporous silica SBA-15 modified in carbon-paste electrode as a sensitive voltammetric sensor for detection of oxalic acid. Sens Actuators B 207:291–296
Ma L, Zeng Q, Zhang M, Wang L, Cheng F (2016) Direct determination of oxalic acid by a bare platinum electrode contrasting a platinum nanoparticles-modified glassy carbon electrode. J Experim Nanosci 11:1242–1252
Rostami S, Azizi SN, Ghasemi S (2017) Preparation of an efficient electrocatalyst for oxalic acid oxidation based on Ag-doped ZSM-5 nanozeolites synthesized from bagasse. J Electroanal Chem 788:235–245
Kesavan L, Kalekar AM, Damlin P, Kvarnström C (2019) Reduced graphene oxide supported palladium nano-shapes for electro-oxidation of oxalic acid. J Electroanal Chem 847:113167
Nagarajan RD, Sundramoorthy AK (2019) One-pot electrosynthesis of silver nanorods/graphene nanocomposite using 4-sulphocalix[4]arene for selective detection of oxalic acid. Sens Actuators B 301:127132
Dodevska T, Shterev I (2020) Electrochemical non-enzymatic sensing of oxalic acid based on PdPt-modified electrodes: application to the analysis of vegetable samples. Monatsh Chem 151:495–504
Omar MN, Salleh AB, Lim HN, Tajudin AA (2016) Electrochemical detection of uric acid via uricase-immobilized graphene oxide. Anal Biochem 509:135–141
Zhang X, Zhang Y-C, Ma L-X (2016) One-pot facile fabrication of graphene-zinc oxide composite and its enhanced sensitivity for simultaneous electrochemical detection of ascorbic acid, dopamine and uric acid. Sens Actuators B 227:488–496
Wang J, Yang B, Zhong J, Yan B, Zhang K, Zhai C, Shiraishi Y, Du Y, Yang P (2017) Dopamine and uric acid electrochemical sensor based on a glassy carbon electrode modified with cubic Pd and reduced graphene oxide nanocomposite. J Colloid Interfac Sci 497:172–180
Rahman MM, Lopa NS, Ju MJ, Lee J-J (2017) Highly sensitive and simultaneous detection of dopamine and uric acid at graphene nanoplatelet-modified fluorine-doped tin oxide electrode in the presence of ascorbic acid. J Electroanal Chem 792:54–60
Aparna TK, Sivasubramanian R, Dar MA (2018) One-pot synthesis of Au-Cu2O/rGO nanocomposite based electrochemical sensor for selective and simultaneous detection of dopamine and uric acid. J Alloy Compd 741:1130–1141
dos Santos PL, Katic V, Toledo KCF, Bonacin JA (2018) Photochemical one-pot synthesis of reduced graphene oxide/prussian blue nanocomposite for simultaneous electrochemical detection of ascorbic acid, dopamine, and uric acid. Sens Actuators B 255:2437–2447
Lal R, Bhatti MA, Shahzad G, Tahira A, Panhwar M, Lal B, Nafady A, Ibupoto ZH (2021) Chemically coupled multiwall carbon nanotubes with leaf-like nanostructures of NiO for sensitive and selective determination of uric acid. J Electron Mater 50:2852–2859
Erdogan ZO, Kucukkolbası S (2021) Fabrication of an electrochemical biosensor based on Fe3O4 nanoparticles and uricase modified carbon paste electrode for uric acid determination. Monatsh Chem 152:309–314
Yang M, Wang H, Liu P, Cheng J (2021) A 3D electrochemical biosensor based on Super-Aligned Carbon NanoTube array for point-of-care uric acid monitoring. Biosens Bioelectr 179:113082
Acknowledgements
The authors thank CMM (Centro de Microscopia e Microanálise—UFRGS) and CNANO (Centro de Nanociência e Nanotecnologia—UFRGS) for the use of microscope and XRD equipment. The authors also thank the CBMM (Companhia Brasileira de Mineração e Metalurgia), which provided the NbCl5 compound.
Funding
The work was financially supported by CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico, FAPERGS (Fundação de Amparo à Pesquisa do estado do Rio Grande do Sul) and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Consent to participate
All authors agree to participate in this work.
Consent for publication
All authors agree on the content and its publication.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Souza, L.V., Virgili, A.H., Teixeira, G.O. et al. Mesoporous structured silica modified with niobium oxide and cobalt hematoporphyrin applied to the simultaneous electrochemical evaluation of oxalic and uric acids. J Sol-Gel Sci Technol 102, 18–29 (2022). https://doi.org/10.1007/s10971-021-05638-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-021-05638-3