Abstract
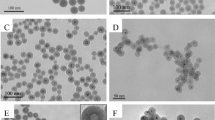
Monodispersed Ag@SiO2 core-shell structure nanoparticles (NPs) are synthesized at room temperature via a one-pot sol–gel synthesis method, and the synthesizing procedure is observed over reaction time in order to investigate the formation mechanism. Ostwald ripening of silver (Ag) core NPs and silica (SiO2) shells during synthesis of core-shell NPs proceeds in parallel to reaction time. Uniform and stable Ag@SiO2 core-shell NPs are formed at 48 h of reaction time. The hydrolysis rate of tetraethyl orthosilicate (TEOS) plays a critical role in the growth of Ag NPs and the final structure of Ag@SiO2 core-shell NPs. The formation mechanisms of single-core and multicore Ag@SiO2 core-shell NPs are discussed in relation to synchronous Ag core and SiO2 shell growth. Optimal reaction conditions for critical parameters (water volume and TEOS and ascorbic acid concentrations) are also determined.

One-pot synthesis of Ag@SiO2 core-shell nanoparticles based on synchronous growth of Ag core and SiO2 shell.
Highlights
-
Synchronous growth mechanism of Ag core and SiO2 shell for monodispersed Ag@SiO2 core-shell nanoparticles was suggested on the base of Ostwald ripening theory.
-
Optimized synthetic parameters for monodispersed Ag@SiO2 core-shell nanoparticles.
-
Uniform and stable Ag@SiO2 core-shell NPs were formed at 48 h of reaction time when the hydrolysis rate of TEOS is moderate.
-
Ag@SiO2 core-shell nanoparticles with multicores were formed at a slower hydrolysis rate, and the Ag@SiO2 core-shell nanoparticles with different core sizes were formed at a faster hydrolysis rate.









Similar content being viewed by others
References
Dhanalekshmi KI, Meena KS (2014) Comparison of antibacterial activities of Ag@TiO2 and Ag@SiO2 core-shell nanoparticles. Spectrochim Acta A Mol Biomol Spectrosc 128:887–890. https://doi.org/10.1016/j.saa.2014.02.063
Gangishetty MK, Scott RW, Kelly TL (2016) Thermal degradation mechanism of triangular Ag@SiO2 nanoparticles. Dalton Trans 45(24):9827–9834. https://doi.org/10.1039/c6dt00169f
Liu C, Li J, Wang J, Qi J, Fan W, Shen J, Sun X, Han W, Wang L (2015) Synthesis of Ag@SiO2 yolk–shell nanoparticles for hydrogen peroxide detection. RSC Adv 5(23):17372–17378. https://doi.org/10.1039/c4ra16061d
Shanthil M, Fathima H, George Thomas K (2017) Cost-effective plasmonic platforms: glass capillaries decorated with Ag@SiO2 nanoparticles on inner walls as SERS substrates. ACS Appl Mater Interfaces 9(23):19470–19477. https://doi.org/10.1021/acsami.6b12478
Zhao K, Rong G, Hao Y, Yu L, Kang H, Wang X, Wang X, Jin Z, Ren Z, Li Z (2016) IgA response and protection following nasal vaccination of chickens with Newcastle disease virus DNA vaccine nanoencapsulated with Ag@SiO2 hollow nanoparticles. Sci Rep. 6:25720. https://doi.org/10.1038/srep25720
Thomas R, Sreejith S, Joshi H, Pedireddy S, Stuparu MC, Zhao Y, Boon SC (2016) Optically induced structural instability in gold–silica nanostructures: a case study. J Phys Chem C 120(20):11230–11236
Fuertes G, Sanchez-Munoz OL, Pedrueza E, Abderrafi K, Salgado J, Jimenez E (2011) Switchable bactericidal effects from novel silica-coated silver nanoparticles mediated by light irradiation. Langmuir 27(6):2826–2833. https://doi.org/10.1021/la1045282
Jimenez-Villar E, Mestre V, de Oliveira PC, de Sa GF (2013) Novel core-shell (TiO2@Silica) nanoparticles for scattering medium in a random laser: higher efficiency, lower laser threshold and lower photodegradation. Nanoscale 5(24):12512–12517. https://doi.org/10.1039/c3nr03603k
Liu S, Zhang Z, Han M (2005) Gram-scale synthesis and biofunctionalization of silica-coated silver nanoparticles for fast colorimetric DNA detection. Anal Chem 77(8):2595–2600
Kobayashi Y, Katakami H, Mine E, Nagao D, Konno M, Liz-Marzan LM (2005) Silica coating of silver nanoparticles using a modified Stober method. J Colloid Interface Sci 283(2):392–396. https://doi.org/10.1016/j.jcis.2004.08.184
Xu K, Wang J-X, Kang X-L, Chen J-F (2009) Fabrication of antibacterial monodispersed Ag–SiO2 core–shell nanoparticles with high concentration. Mater Lett 63(1):31–33. https://doi.org/10.1016/j.matlet.2008.08.039
Niitsoo O, Couzis A (2011) Facile synthesis of silver core—silica shell composite nanoparticles. J Colloid Interface Sci 354(2):887–890. https://doi.org/10.1016/j.jcis.2010.11.013
Bastakoti BP, Guragain S, Yusa S-i, Nakashima K (2012) Novel synthesis route for Ag@SiO2 core–shell nanoparticles via micelle template of double hydrophilic block copolymer. RSC Adv 2(14). https://doi.org/10.1039/c2ra20316b
Zhang Y, Kong X, Xue B, Zeng Q, Liu X, Tu L, Liu K, Zhang H (2013) A versatile synthesis route for metal@SiO2 core–shell nanoparticles using 11-mercaptoundecanoic acid as primer. J Mater Chem C 1(39). https://doi.org/10.1039/c3tc31171f
Viazmitinov D, Matyushkin L, Maximov A (2014) Synthesis of core-shell Ag/SiO2 nanoparticles for SPASER structures. J Phys 1:012015
Lismont M, Paez CA, Dreesen L (2015) A one-step short-time synthesis of Ag@SiO2 core-shell nanoparticles. J Colloid Interface Sci 447:40–49. https://doi.org/10.1016/j.jcis.2015.01.065
Li Z, Jia L, Li Y, He T, Li X-M (2015) Ammonia-free preparation of Ag@SiO2 core/shell nanoparticles. Appl Surf Sci 345:122–126. https://doi.org/10.1016/j.apsusc.2015.03.159
Karimipour M, Shabani E, Molaei M (2015) Rapid synthesis of Ag nanoparticles Ag@SiO2 core–shells. Int J Mater Res 106(5):532–534
Alimunnisa J, Ravichandran K, Meena KS (2017) Synthesis and characterization of Ag@SiO2 core-shell nanoparticles for antibacterial and environmental applications. J Mol Liq 231:281–287. https://doi.org/10.1016/j.molliq.2017.01.103
Casavola M, Buonsanti R, Caputo G, Cozzoli PD (2008) Colloidal strategies for preparing oxide-based hybrid nanocrystals. Eur J Inorg Chem 2008(6):837–854. https://doi.org/10.1002/ejic.200701047
Han L, Wei H, Tu B, Zhao D (2011) A facile one-pot synthesis of uniform core–shell silver nanoparticle@mesoporous silica nanospheres. Chem Commun 47(30):8536–8538. https://doi.org/10.1039/C1CC12718G
Saint-Cricq P, Wang J, Sugawara-Narutaki A, Shimojima A, Okubo T (2013) A new synthesis of well-dispersed, core–shell Ag@SiO2 mesoporous nanoparticles using amino acids and sugars. J Mater Chem B 1(19):2451–2454
Karimipour M, Mostoufirad S, Molaei M, Nikabadi H, Nesheli A (2016) Free reducing agent, one pot, and two steps synthesis of Ag@SiO2 core-shells using microwave irradiation. J Nano-Electron Phys 8(3):03020-1–03020-4
Otari S, Yadav H, Thorat N, Patil R, Lee J, Pawar S (2016) Facil one pot synthesis core shell Ag@SiO2 nanoparticles for catalytic and antimicrobial activity. Mater Lett 167:179–182
Li C, Wang C, Ji Z, Jiang N, Lin W, Li D (2018) One-pot synthesis of silver@ silica core–shell nanospheres and their application in optical limiting materials. Appl Phys A 124(3):244
Qian C (2018) Facile synthesis of hollow porous silica micro-and nanospheres and their application. Aalto University publication series DOCTORAL DISSERTATIONS, 179/2018
Chen Q, Ge Y, Granbohm H, Hannula S-P (2018) Effect of ethanol on Ag@ mesoporous silica formation by in situ modified Stöber method. Nanomaterials 8(6):362
Jang L-W, Jeon D-W, Sahoo T, Jo D-S, Ju J-W, Lee S-j, Baek J-H, Yang J-K, Song J-H, Polyakov AY (2012) Localized surface plasmon enhanced quantum efficiency of InGaN/GaN quantum wells by Ag/SiO 2 nanoparticles. Opt Express 20(3):2116–2123
Jang L-W, Jeon D-W, Sahoo T, Polyakov AY, Saravanakumar B, Yu Y-T, Cho Y-H, Yang J-K, Lee I-H (2012) Energy coupling processes in InGaN/GaN nanopillar light emitting diodes embedded with Ag and Ag/SiO2 nanoparticles. J Mater Chem 22(40):21749–21753
Sakthisabarimoorthi A, Dhas SMB, Jose M (2018) Nonlinear optical properties of Ag@SiO2 core-shell nanoparticles investigated by continuous wave He-Ne laser. Mater Chem Phys 212:224–229
Raj S, Rai P, Yu Y-T (2014) Pulse electrophoresis deposition of Ag@SiO2 core–shell nanoparticles on FTO substrate. Mater Lett 117:116–119. https://doi.org/10.1016/j.matlet.2013.11.123
Liz-Marzán LM, Giersig M, Mulvaney P (1996) Synthesis of nanosized gold− silica core− shell particles. Langmuir 12(18):4329–4335
Lu H, Ju H, Yang Q, Li Z, Ren H, Xin X, Xu G (2013) Synthesis of Ag@SiO2 hybrid nanoparticles templated by a Triton X-100)/1-hexanol/cyclohexane/H2O water-in-oil microemulsion. CrystEngComm 15(33). https://doi.org/10.1039/c3ce40432c
Paredes D, Ortiz C, Torres R (2014) Synthesis, characterization, and evaluation of antibacterial effect of Ag nanoparticles against Escherichia coli O157: H7 and methicillin-resistant Staphylococcus aureus (MRSA). Int J Nanomed 9:1717
Fang Z, Li S, Gong Y, Liao W, Tian S, Shan C, He C (2014) Comparison of catalytic activity of carbon-based AgBr nanocomposites for conversion of CO2 under visible light. J Saudi Chem Soc 18(4):299–307
Kaur B, Srivastava R, Satpati B (2015) Silver nanoparticle decorated polyaniline–zeolite nanocomposite material based non-enzymatic electrochemical sensor for nanomolar detection of lindane. RSC Adv 5(71):57657–57665
Chen R, Nuhfer NT, Moussa L, Morris HR, Whitmore PM (2008) Silver sulfide nanoparticle assembly obtained by reacting an assembled silver nanoparticle template with hydrogen sulfide gas. Nanotechnology 19(45):455604
Sentosun K, Sanz Ortiz MN, Batenburg KJ, Liz-Marzán LM, Bals S (2015) Combination of HAADF-STEM and ADF-STEM tomography for core–shell hybrid materials. Part Part Syst Charact 32(12):1063–1067. https://doi.org/10.1002/ppsc.201500097
Lee J, Park JC, Song H (2008) A nanoreactor framework of a Au@SiO2 yolk/shell structure for catalytic reduction ofp-nitrophenol. Adv Mater 20(8):1523–1528. https://doi.org/10.1002/adma.200702338
Mine E, Yamada A, Kobayashi Y, Konno M, Liz-Marzán LM (2003) Direct coating of gold nanoparticles with silica by a seeded polymerization technique. J Colloid Interface Sci 264(2):385–390. https://doi.org/10.1016/s0021-9797(03)00422-3
Kobayashi Y, Inose H, Nakagawa T, Gonda K, Takeda M, Ohuchi N, Kasuya A (2013) Synthesis of Au–silica core–shell particles by sol–gel process. Surf Eng 28(2):129–133. https://doi.org/10.1179/1743294411y.0000000069
Singha D, Barman N, Sahu K (2014) A facile synthesis of high optical quality silver nanoparticles by ascorbic acid reduction in reverse micelles at room temperature. J Colloid Interface Sci 413:37–42
Anastas PT, Warner JC (2000) Green chemistry. Oxford University Press, Oxford, p 135. https://doi.org/10.1021/op000054t
Xiong J, Wang Y, Xue Q, Wu X (2011) Synthesis of highly stable dispersions of nanosized copper particles using L-ascorbic acid. Green Chem 13(4):900–904
Zhao N, Wei Y, Sun N, Chen Q, Bai J, Zhou L, Qin Y, Li M, Qi L (2008) Controlled synthesis of gold nanobelts and nanocombs in aqueous mixed surfactant solutions. Langmuir 24(3):991–998
Wang Y, Camargo PH, Skrabalak SE, Gu H, Xia Y (2008) A facile, water-based synthesis of highly branched nanostructures of silver. Langmuir 24(20):12042–12046
Song Y, Garcia RM, Dorin RM, Wang H, Qiu Y, Shelnutt JA (2006) Synthesis of platinum nanocages by using liposomes containing photocatalyst molecules. Angew Chem 118(48):8306–8310
Zhang W, Qiao X, Chen J (2007) Synthesis of nanosilver colloidal particles in water/oil microemulsion. Colloids Surf A 299(1-3):22–28
Setua P, Ghatak C, Rao VG, Das S, Sarkar N (2012) Dynamics of solvation and rotational relaxation of coumarin 480 in pure aqueous-AOT reverse micelle and reverse micelle containing different-sized silver nanoparticles inside its core: a comparative study. J Phys Chem B 116(12):3704–3712
Evanoff DD, Chumanov G (2004) Size-controlled synthesis of nanoparticles. 1. “Silver-only” aqueous suspensions via hydrogen reduction. J Phys Chem B 108(37):13948–13956
Lee H, Habas SE, Somorjai GA, Yang P (2008) Localized Pd overgrowth on cubic Pt nanocrystals for enhanced electrocatalytic oxidation of formic acid. J Am Chem Soc 130(16):5406–5407
Suriati G, Mariatti M, Azizan A (2014) Synthesis of silver nanoparticles by chemical reduction method: Effect of reducing agent and surfactant concentration. Int J Automot Mech Eng 10:1920
Aslan K, Leonenko Z, Lakowicz JR, Geddes CD (2005) Fast and slow deposition of silver nanorods on planar surfaces: application to metal-enhanced fluorescence. J Phys Chem B 109(8):3157–3162
Kelly J, Keegan G, Brennan-Fournet M (2012) Triangular silver nanoparticles: their preparation, functionalisation and properties. Acta Phys Polonica A 122(2):337–345
Rathore G, Gupta A, Singh L (2020) An eco-friendly approach for fabrication of silver nanoparticles by using ascorbic acid from various native sources. J Pharm Res 12(2):41–48
Sondi I, Goia DV, Matijević E (2003) Preparation of highly concentrated stable dispersions of uniform silver nanoparticles. J Colloid Interface Sci 260(1):75–81
Anigol LB, Charantimath JS, Gurubasavaraj PM (2017) Effect of Concentration and pH on the Size of silver nanoparticles synthesized by green chemistry. Org Med Chem Int J 3(5):1–5
Zhang W, Zhu L, Ye H, Liu H, Li W (2016) Modifying a waterborne polyacrylate coating with a silica sol for enhancing anti-fogging performance. RSC Adv 6(95):92252–92258. https://doi.org/10.1039/c6ra19237h
Yang Y, Han S, Zhou G, Zhang L, Li X, Zou C, Huang S (2013) Ascorbic-acid-assisted growth of high quality M@ZnO: a growth mechanism and kinetics study. Nanoscale 5(23):11808–11819. https://doi.org/10.1039/C3NR03934J
Acknowledgements
This research was supported by the BK21 plus program of the Ministry of Education and Human-Resource Development, South of Korea; and a National Research Foundation of Korea (NRF) grant funded by the Korean government, South Korea (MSIP) (BRL No. 2015042417, 2016R1A2B4014090).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ahemad, M.J., Yu, YT. Investigating the mechanism of uniform Ag@SiO2 core-shell nanostructures synthesis by a one-pot sol–gel method. J Sol-Gel Sci Technol 96, 679–689 (2020). https://doi.org/10.1007/s10971-020-05392-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-020-05392-y