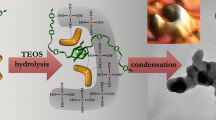
Abstract
This work evaluates the structural evolution of silicate gel over time with mineral additives. A comparison between fresh and aged silicate gel synthesized from silica and potassium hydroxide was carried out using FTIR, Raman, and 29Si MAS-NMR spectroscopies. Then, different additives, including NaOH, AlOOH, Li2B4O7, and HCl, were incorporated into the silica solution. The effects of these additives on the aspect, transparency, Qn polymerization, and stability of the silicate gels were studied. Structural studies showed that the quantification of Qn species was difficult using FTIR and Raman spectroscopies because of the existence of several contributions in the same spectral region of silicate species. More precise information was provided by 29Si NMR spectroscopy, which demonstrated the increase in Q3 species to the detriment of Q4, revealing the depolymerization of the structure over time. Further study of the effect of additives revealed that HCl, AlOOH, and Li2B4O7 did not improve the stability of the gel due to the formation of several networks. However, promising stability and transparency results were obtained using NaOH with a silicate/modifier molar ratio of 2.52.

Highlights
-
The decrease of Q4/Q3 ratio after ageing reveals the depolymerization of the structure of silicate gel.
-
Adding NaOH to the silicate gel preserves the transparency and improves its stability over time.
-
Polymerization of siliceous species with nSi/(nNa + nK) ratio equal to 2.52.







Similar content being viewed by others
References
Nasri Z, Shams E (2009) Application of silica gel as an effective modifier for the voltammetric determination of dopamine in the presence of ascorbic acid and uric acid. Electrochim Acta 54:7416–7421
Hench LL, Vasconcelos W (1990) Gel silica science. Annu Rev Mater Sci 20:269–298
Estella J, Echeverría J, Laguna M, Garrido J (2007) Effects of aging and drying conditions on the structural and textural properties of silica gels. Microporous Mesoporous Mater 102:274–282
Guibal E, Lorenzelli R, Vincent T, Cloirec P (2010) Application of silica gel to metal ion sorption: static and dynamic removal of uranyl ions. Environ Technol 16:101–114
Sui H, Liu H, An P, He L, Li X, Cong S (2017) Application of silica gel in removing high concentrations toluene vapor by adsorption and desorption process. J Taiwan Inst Chem E 74:218–224
Hentzschel M, Alnaief M, Smirnova I, Sakmann A, Leopold CS (2012) Tableting properties of silica aerogel and other silicates. Drug Dev Ind Pharm 38:462–467
Livage J, Henry M, Sanchez C (1988) Sol-gel chemistry of transition metal oxides. Prog Solid St Chem 18:259–341
Vail JG (1952) Soluble silicates: their properties and uses, vol 1. Reinhold, New York, p 357
McGavack J, Patrick WA (1920) The adsorption of sulfur dioxide by the gel of silicic acid. J Am Chem Soc 42:946–978
Gerber T, Himmel B, Hübert C (1994) WAXS and SAXS investigation of structure formation of gels from sodium water glass. J Non-Cryst Solids 175:160–168
Lee CJ, Kim GS, Hyun SH (2002) Synthesis of silica aerogels from waterglass via new modified ambient drying. J Mater Sci 37:2237–2241
Orgel G, Phalippou J, Hench LL(1986) Structural changes of silica xerogels during low temperature dehydration. J Non-Cryst Solids 88(1):114–130
Hench LL, Prassas M, Phalippou P (1982) Preparation of 33 Na2O 37 SiO2 glass by gel glass transformation. J Non-Cryst Solids 53:183–193
Dokkum V, Hulskotte HP, Kramer JHJ, Wilmot KJM (2004) Emission, fate and effects of soluble silicates (waterglass) in the aquatic environment. Environ Sci Technol 38:515–521
Engelhard G, Zeigan D, Jancke H, Hoebbel D, Weiker Z (1975) High resolution 29Si NMR of silicates and Zeolites. Anorg Allg Chem 418:17–28
Brykov AS, Danilov VV, Yu Aleshunina E (2008) State of silicon in silicate and silica-containing solutions and their binding properties. Russ J Appl Chem 81:1717–1721
Tognonvi MT, Soro J, Rossignol S (2012) Physical-chemistry of silica/alkaline silicate interactions during consolidation. Part 2: effect of pH. J Non Cryst Solids 358:492–501
Svensson L, Sjöberg S, Öhman LO (1986) Polysilicate equilibria in concentrated sodium silicate solutions. J Chem Soc Faraday Trans 82:3635–3646
Cannas C, Casu M, Musinu A, Piccaluga G (2005) 29Si CP MAS NMR and Near-IR study of sol-gel microporous silica with tunable surface area. J Non-Cryst Solids 351:34–76
Estella J, Echeverria JC, Laguna M, Garrido J (2007) Effect of supercritical drying conditions in ethanol on the structural and textural properties of silica aerogels. Microporous Mesoporous Mater 15:102–274
Lutz W, Täschner D, Kurzhals R, Heidemann D, Hübert C (2009) Characterization of silica gels by 29Si MAS NMR and IR spectroscopic measurements. Z Anorg Allg Chem 635:2191–2196
MacDonald SA, Schardt CR, Masiello DJ, Simmons JH (2000) Dispersion analysis of FTIR reflection measurements in silicate glasses. J Non-Cryst Solids 275:72–82
Mysen BO, Cody GD (2005) Solution mechanisms of H2O in depolymerized peralkaline melts. Geochim Cosmochim Acta 69:5557–5566
Vidal L, Gharzouni A, Joussein E, Colas M, Cornette J, Absi J, Rossignol S (2017) Determination of the polymerization degree of various alkaline solutions: Raman investigation. J Sol-Gel Sci Technol 83:1–11
Arnoult M, Dupuy C, Colas M, Cornette J, Duponchel L, Rossignol S (2019) Determination of the reactivity degree of various alkaline solutions: a chemometric investigation. Appl Spectrosc 73:12
Malfait WJ, Zakaznova-Herzog VP, Halter WE (2007) Quantitative Raman spectroscopy: High-temperature speciation of potassium silicate melts. J Non-Cryst Solids 353:4029–4042
Kalapathy U, Proctor A, Shultz J (2000) Production and properties of flexible sodium silicate films from rice hull ash silica. Bioresour Technol 72:99–106
Wijnen PWJG, Beelen TPM, Rummens KPJ, Saeijs HCPL, De Haan JW, Van De Ven LJM, Van Santen RA (1991) The molecular basis of aging of aqueous silica gel. J Colloid Interf Sci 145:17–32
Nassif N, Roux C, Coradin T, Rager MN, Bouvet OMM, Livage JA (2003) Sol-gel matrix to preserve the viability of encapsulated bacteria. J Mater Chem 13:203–208
Murakata T, Sato S, Ohgawara T (1992) Control of pore size distribution of silica gel through sol-gel process using inorganic salts and surfactants as additives. J Mater Sci 27:1567–1574
Jiang ZH, Zhang QY (2014) The structure of glass: a phase equilibrium approach. Prog Mater Sci 61:144–215
Neyret M, Lenoir M, Granhean A, Massoni N, Penelon B, Malki M (2015) Ionic transport of alkali in borosilicate glass. Role of alkali nature on glass structure and on ionic conductivity at the glassy state. J Non-Cryst Solids 410:74–81
Xie J, Tang H, Wang J, Wu M, Han J, Liu C (2018) Network connectivity and properties of non-alkali aluminoborosilicate glasses. J Non-Cryst Solids 481:403–408
Deilmann L, Winter O, Cerrutti B, Bradtmüller H, Herzig C, Limbeck A, Lahayne O, Hellmich C, Eckert H, Eder D (2020) Effect of boron incorporation on the bioactivity, structure, and mechanical properties of ordered mesoporous bioactive glasses. Mater Chem B 8:1456
Fang YT, Liu T, Zhang ZC, Gao XN (2014) Silica gel adsorbents doped with Al, Ti, and Co ions improved adsorption capacity, thermal stability and aging resistance. Renew Energy 63:755–761
Liu S, Boffa V, Yang D, Fan Z, Meng F, Yue Y (2018) Clarifying the gel-to-glass transformation in Al2O3-SiO2 systems. J Non-Cryst Solids 492:77–83
Ren JJ, Zhang L, Eckert H (2014) Medium-range order in sol–gel prepared Al2O3–SiO2glasses: new results from solid-state NMR. J Phys Chem C 118:4906–4917
Szu SP, Klein LC, Greenblatt M (1990) Effect of precursors on lithium containing silicate gels studied by 7Li nuclear magnetic resonance. J Non-Cryst Solids 121:1–3
Schwartz I, Anderson P, de Lambilly H, Klein LC (1986) Stability of lithium silicate gels. J Non-Cryst Solids 83:391–399
Dimas D, Giannopoulou I, Panias D (2009) Polymerization in sodium silicate solutions: a fundamental process in geopolymerization technology. J Mater Sci 44:3719–3730
Prassas M, Phalippou J, Hench LL (1984) Preparation of xNa2O(1-x)SiO2 gels for the glass process II the gel-glas transition. J Non-Cryst Solids 63(1):375–389
Muroya M (1999) Correlation between the formation of silica skeleton and Fourier transform reflection infrared absorption spectroscopy spectra. Colloid Surf A 157:147–155
Chiang CH, Ishida H, Koenig JL (1980) The structure of y-Aminopropyltriethoxysilane on glass surfaces. J Colloid Interface Sci 74:396–404
Lutz W, Taschner D, Kurzhals R, Heidemann D, Hubert C (2009) Characterization of silica gels by 29Si MAS NMR and IR spectroscopic measurements. Z Anorg Allg Chem 635:2191–2196
Tan J, Zhao S, Wang W, Davies G, Mo X (2004) The effect of cooling rate on the structure of sodium silicate glass. Mater Sci Eng B 106:295–299
Knight CTG (1988) A two-dimensional silicon-29 nuclear magnetic resonance spectroscopic study of the structure of the silicate anions present in an aqueous potassium silicate solution. J Chem Soc Dalton Trans 1457–1460
Kawazoe H (1994) Application of NMR spectroscopy to the science and technology of glasses and ceramics. In: Ando I, Webb GA (eds) Annual reports on NMR spectroscopy, vol 28, Academic Press, p 1–27
Sjöberg S (1996) Silica in aqueous environments. J Non-Cryst Solids 196:51–57
Michaud PT, Babic D (1998) A Raman study of etching silicon in aqueous tetramethylammonium hydroxide. J Electrochem Soc 145:11
Gryniewicz-Ruzicka C, Arzhantsev S, Pelster LN, Westenberger B, Buhse J, Kauffman (2011) Multivariate calibration and instrument standardization for the rapid detection of diethylene glycol in glycerien by Raman spectroscopy. Appl Spectrosc 65:334–341
Mendelovici E, Frost RL, Kloprogge T (2000) Cryogenic Raman spectroscopy of glycerol. J Raman Spectrosc 31:1121–1126
Zotov N, Keppler H (1998) The influence of water on the structure of hydrous sodium tetrasilicate glasses. Am Mineral 83:823–834
Matos MC, Ilharco LM, Almeida RM (1992) The evolution of TEOS to silica gel and glass by vibrational spectroscopy. J Non-Cryst Solids 147-148:232–237
Brykov AS, Danilov VV, Aleshunina EY (2008) State of silicon in silicate and silica-containing solutions and their binding properties. Russ J Appl Chem 81:1717–1721
Melkior T, Yahiaoui S, Thoby D, Motellier S, Barthès V (2007) Diffusion coefficients of alkaline cations in Bure mudrock. Phys Chem Earth 32:453–462
Goudarzi N (2013) Silicon-29 NMR spectroscopy study of the effect of tetraphenylammonium (TPA) as a template on distribution of silicate species on alkaline aqueous and alcoholic silicate solutions. Appl Magn Reson 44:469–478
Vega AJ, Scherer GW (1989) Study of structural evolution of silica gel using 1H and 29Si NMR. J Non-Cryst Solids 111:153–166
Goto K (1956) Effect of pH on polymerization of silicic acid. J Phys Chem 60:1007–1008
Bangi UKH, Jung IK, Park CS, Baek S, Park HH (2013) Optically transparent silica aerogels based on sodium silicate by a two step sol–gel process and ambient pressure drying. Solid State Sci 18:50–57
Panias D, Asimidis P, Paspaliaris I (2001) Solubility of boehmite in concentrated sodium hydroxide solutions: model development and assessment. Hydrometallurgy 59:15–29
Dupuy C, Gharzouni A, Sobrados I, Texier-Mandoki N, Bourbon X, Rossignol S (2019) 29Si, 27Al, 31P and 11B magic angle spinning nuclear magnetic resonance study of the structural evolutions induced by the use of phosphor- and boron–based additives in geopolymer mixtures. J Non-Cryst Solids 521:119–541
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
El Felss, N., Gharzouni, A., Colas, M. et al. Structural study of the effect of mineral additives on the transparency, stability, and aging of silicate gels. J Sol-Gel Sci Technol 96, 265–275 (2020). https://doi.org/10.1007/s10971-020-05385-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-020-05385-x