Abstract
The phase separation process in silica-based gelling systems in the presence of a polymer (polyethylene oxide, PEO) is shown to result in the formation of porous materials with the open-cell foam structure. The PEO concentration range in the reaction mixture where these foam-like structures are observed is determined. It is shown that phase separation begins with the formation of discrete droplets of solvent in the gel phase similar to the phase separation in some polymerizing organic systems. Further, liquid droplets grow and coalesce that leads to the formation of a system of interconnected macropores with a shape close to the spherical one. The macropore sizes are shown to depend on the PEO content, but no significant difference in the mesoporosity and surface area of the monoliths is observed. Dried and calcined porous silica monoliths are obtained with macropore sizes from 0.8 to 41 μm. The maximal compressive strength is 2.2 MPa, porosity is 85%, permeability coefficient is 3.5·10−12 m2, and the specific surface area is 210 m2 g−1. The materials can be used as supports in flow-through catalytic systems.

Highlights
-
Macroporous open-cell foams are formed in silica-based gelling systems.
-
In the presence of PEO, phase separation in silica gel occurs as in organic polymers.
-
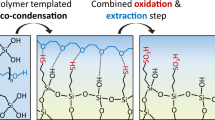
PEO macromolecules are connected with the silica oligomers before and after phase separation.
-
The macropore sizes are determined by the polymer content in the reaction mixture.








Similar content being viewed by others
References
Tanimu A, Jaenicke S, Alhooshani K (2017) Heterogeneous catalysis in continuous flow microreactors: a review of methods and applications. Chem Eng J 327:792–821. https://doi.org/10.1016/j.cej.2017.06.161
Cybulski A, Moulijn JA (1994) Monoliths in heterogeneous catalysis. Catal Rev Sci Eng 36(2):179–270. https://doi.org/10.1080/01614949408013925
Galarneau A, Sachse A, Said B, Pelisson C-H, Boscaro P, Brun N, Courtheoux L, Olivi-Tran N, Coasne B, Fajula F (2016) Hierarchical porous silica monoliths: a novel class of microreactors for process intensification in catalysis and adsorption. C R Chim 19:231–247. https://doi.org/10.1016/j.crci.2015.05.017
Enke D, Glӓser R, Tallarek U (2016) Sol-gel and porous glass-based silica monoliths with hierarchical pore structure for solid-liquid catalysis. Chem Ing Tech 88(11):1561–1585. https://doi.org/10.1002/cite.201600049
Italiano C, Ashraf MA, Pino L, Moncada Quintero CW, Specchia S, Vita A (2018) Rh/CeO2 thin catalytic layer deposition on alumina foams: catalytic performance and controlling regimes in biogas reforming processes. Catalysts 8(10):448. https://doi.org/10.3390/catal8100448
Twigg MV, Richardson JT (2007) Fundamentals and applications of structured ceramic foam catalysts. Ind Eng Chem Res 46(12):4166–4177. https://doi.org/10.1021/ie061122o
Voltolina S, Marín P, Díez FV, Ordóñez S (2017) Open-cell foams as beds in multiphase reactors: residence time distribution and mass transfer. Chem Eng J 316:323–331. https://doi.org/10.1016/j.cej.2017.01.113
Yang X-Y, Chen L-H, Li Y, Rooke JC, Sanchezc C, Su B-L (2017) Hierarchically porous materials: synthesis strategies and structure design. Chem Soc Rev 46:481–558. https://doi.org/10.1039/C6CS00829A
Blin J-L, Léonard A, Yuan Z-Y, Gigot L, Vantomme A, Cheetham AK, Su BL (2003) Hierarchically mesoporous/macroporous metal oxides templated from polyethylene oxide surfactant assemblies. Angew Chem Int Ed 42:2872–2875. https://doi.org/10.1002/anie.200250816
Okay O (2000) Macroporous copolymer networks. Prog Polym Sci 25:711–779. https://doi.org/10.1016/S0079-6700(00)00015-0
Kim S-H, Shin C-K, Ahn C-H, Kim G-J (2006) Syntheses and application of silica monolith with bimodal meso/macroscopic pore structure. J Porous Mater 13(3–4):201–205. https://doi.org/10.1007/s10934-006-8005-6
Feinle A, Elsaesser MS, Hüsing N (2016) Sol-gel synthesis of monolithic materials with hierarchical porosity. Chem Soc Rev 45:3377–3399. https://doi.org/10.1039/C5CS00710K
Izaak TI, Vodyankina OV (2009) Macroporous monolithic materials: synthesis, properties and application. Russ Chem Rev 78(1):77–88. https://doi.org/10.1070/RC2009v078n01ABEH003892
Fletcher PDI, Haswell SJ, He P, Kelly SM, Mansfield A (2011) Permeability of silica monoliths containing micro- and nano-pores. J Porous Mater 18:501–508. https://doi.org/10.1007/s10934-010-9403-3
Galarneau A, Abid Z, Said B, Didi Y, Szymanska K, Jarzębski A, Tancret F, Hamaizi H, Bengueddach A, Renzo FD, Fajula F (2016) Synthesis and textural characterization of mesoporous and meso-/macroporous silica monoliths obtained by spinodal decomposition. Inorganics 4(2):9. https://doi.org/10.3390/inorganics4020009.
Izaak TI, Martynova DO, Stonkus OA, Slavinskaya EM, Boronin AI (2013) Deposition of silver nanoparticles into porous system of sol-gel silica monoliths and properties of silver/porous silica composites. J Sol-Gel Sci Technol 68:471–478. https://doi.org/10.1007/s10971-013-2977-x
Triantafillidis C, Elsaesser MS, Hüsing N (2013) Chemical phase separation strategies towards silica monoliths with hierarchical porosity. Chem Soc Rev 42:3833–3846. https://doi.org/10.1039/c3cs35345a
Nakanishi K (1997) Pore structure control of silica gels based on phase separation. J Por Mater 4:67–112. https://doi.org/10.1023/A:1009627216939
Nakanish K, Soga N (1992) Phase separation in silica sol-gel system containing polyacrylic acid I. Gel formation behavior and effect of solvent composition. J Non-Cryst Solids 139:1–13. https://doi.org/10.1016/S0022-3093(05)80800-2
Nakanishi K (2011) Monolithic silicas in separation science. WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
Lemaire A, Rooke JC, Chen L-H, Su B-L (2011) Direct observation of macrostructure formation of hierarchically structured meso-macroporous aluminosilicates with 3D interconnectivity by optical microscope. Langmuir 27:3030–3043. https://doi.org/10.1021/la104679h
Nakanishi K, Takahashi R, Nagakane T, Kitayama K, Koheiya N, Shikata H, Soga N (2000) Formation of hierarchical pore structure in silica gel. J Sol-Gel Sci Technol 17:191–210. https://doi.org/10.1023/A:1008707804908
Serrano A, Martín del Campo J, Peco N, Rodriguez JF, Carmona M (2019) Influence of gelation step for preparing PEG–SiO2 shape-stabilized phase change materials by sol-gel method. J Sol-Gel Sci Technol 89(3):731–742. https://doi.org/10.1007/s10971-018-4866-9
Preari M, Spinde K, Lazic J, Brunner E, Demadis KD (2014) Bioinspired insights into silicic acid stabilization mechanisms: The dominant role of polyethylene glycol-induced hydrogen bonding. J Am Chem Soc 136:4236–4244. https://doi.org/10.1021/ja411822s
Niznansky D, Rehspringer JL (1995) Infrared study of SiO2 sol to gel evolution and gel aging. J Non-Cryst Solids 180:191–196. https://doi.org/10.1016/0022-3093(94)00484-6
Koganti VR, Das S, Rankin SE (2014) In situ FTIR investigation of the kinetics of silica polycondensation in surfactant templated, mesostructured thin films. J Phys Chem C 118:19450–19461. https://doi.org/10.1021/jp505651j
Al-Oweini R, El-Rassy H (2009) Synthesis and characterization by FTIR spectroscopy of silica aerogels prepared using several Si(OR)4 and R”Si(OR’)3 precursors. J Mol Struct 919:140–145. https://doi.org/10.1016/j.molstruc.2008.08.025
Tanaka H (2000) Viscoelastic phase separation. J Phys: Condens Matter 12:207–264. https://doi.org/10.1088/0953-8984/12/15/201
Tanaka H (1994) Critical dynamics and phase‐separation kinetics in dynamically asymmetric binary fluids: New dynamic universality class for polymer mixtures or dynamic crossover? J Chem Phys 100(7):5323–5337. https://doi.org/10.1063/1.467197
Tanaka H, Nishikawa Y, Koyama T (2005) Network-forming phase separation of colloidal suspensions. J Phys: Condens Matter 17:143–153. https://doi.org/10.1088/0953-8984/17/15/L02
Saito H, Kanamori K, Nakanishi K, Hirao K, Nishikawa Y, Jinnaic H (2007) Three-dimensional observation of macroporous silica gels and the study on structural formation mechanism. Colloids Surf A 300(1–2):245–252. https://doi.org/10.1016/j.colsurfa.2006.12.075
Stoeckel D, Kübel C, Loeh MO, Smarsly BM, Tallarek U (2015) Morphological analysis of physically reconstructed silica monoliths with submicrometer macropores: effect of decreasing domain size on structural homogeneity. Langmuir 31:7391–7400. https://doi.org/10.1021/la5046018
Motokawa M, Kobayashi H, Ishizuka N, Minakuchi H, Nakanishi K, Jinnai H, Hosoya K, Ikegami T, Tanaka N (2002) Monolithic silica columns with various skeleton sizes and through-pore sizes for capillary liquid chromatography. J Chromatogr A 961:53–63. https://doi.org/10.1016/S0021-9673(02)00133-4
Hara T, Desmet G, Baron GV, Minakuchi H, Eeltink S (2016) Effect of polyethylene glycol on pore structure and separation efficiency of silica-based monolithic capillary columns. J Chromatogr A 1442:42–52. https://doi.org/10.1016/j.chroma.2016.03.009
Style RW, Sai T, Fanelli N, Ijavi M, Smith-Mannschott K, Xu Q, Wilen LA, Dufresne ER (2018) Liquid-liquid phase separation in an elastic network. Phys Rev X 8(1):1–9. https://doi.org/10.1103/PhysRevX.8.011028
Gent AN, Wang C (1991) Fracture mechanics and cavitation in rubber-like solids. J Mater Sci 26(3392):29–34. https://doi.org/10.1007/BF01124691
Ball JM (1982) Discontinuous equilibrium solutions and cavitation in nonlinear elasticity. Philos Trans R Soc A 306(1496):557–611. https://doi.org/10.1098/rsta.1982.0095
Gent AN (1974) Rubber and rubber elasticity: a review. J Polym Sci Polym Symp 48(1):1–17. https://doi.org/10.1002/polc.5070480104
Kohns R, Haas CP, Höltzel A, Splith C, Enke D, Tallarek U (2018) Hierarchical silica monoliths with submicron macropores as continuous-flow microreactors for reaction kinetic and mechanistic studies in heterogeneous catalysis. React Chem Eng 3:353–364. https://doi.org/10.1039/c8re00037a
Acknowledgements
This work was supported by Russian Science Foundation (project no. 19-73-30026). The authors acknowledge Dr M.A. Salaev for language review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Vodorezova, O.Y., Lapin, I.N. & Izaak, T.I. Formation of the open-cell foam structures in tetraethoxysilane-based gelling systems. J Sol-Gel Sci Technol 94, 384–392 (2020). https://doi.org/10.1007/s10971-020-05244-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-020-05244-9



