Abstract
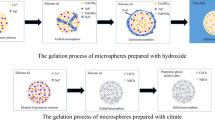
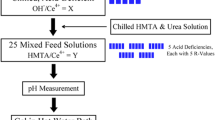
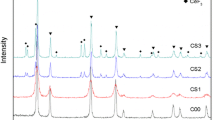
Cerium dioxide microspheres were successfully prepared by internal gelation with cerium citrate as the precursor. The effects of solution containing hexamethylenetetramine (HMTA) and urea on the stability of precursor solution and on the sintered microspheres were investigated. The results indicate that the gelled microspheres were composed of [CeCit∙xH2O]. Propylene glycol methyl ether was a good solvent for solvothermal treatment. The solution containing HMTA and urea had an important effect on the stability of precursor solution; it also greatly affected the surface morphology, specific surface area, pore volume, and average pore diameter of microspheres. The porous CeO2 microspheres were obtained by sintering at 600 ℃ for 2 h. CeO2 microspheres prepared with cerium citrate as precursor had a larger pore volume than microspheres prepared with cerium hydroxide as the precursor.

Highlights
-
Porous CeO2 microspheres have been prepared with [CeCit∙xH2O] as the precursor.
-
Propylene glycol methyl ether was a good solvent for solvothermal treatment of gel microspheres.
-
The H-U solution greatly affected the surface morphology, specific surface area, pore volume, and average pore diameter of microspheres.
-
Compared with sintered microspheres prepared by cerium hydroxide, sintered microspheres prepared by cerium citrate have larger pore volume.













Similar content being viewed by others
References
Osaka M, Miwa S, Tachi Y (2006) Simple fabrication process for CeO2–MgO composite as surrogate for actinide-containing target for use in nuclear fuel. Ceram Int 32:659–663
Haas D, Fernandez A, Na¨stren C, Staicu D, Somers J, Maschek W, Chen X (2006) Properties of cermet fuels for minor actinides transmutation in ADS. Energ Convers Manag 47:2724–2731
Haas D, Fernandez A, Staicu D, Somers J, Maschek W, Liu P, Chen X (2008) CERMET fuel behavior and properties in ADS reactors. Energ Convers Manag 49:1928–1933
Oigawa H, Tsujimoto K, Nishihara K, Sugawara T, Kurata Y, Takei H, Saito S, Sasa T, Obayashi H (2011) Role of ADS in the back-end of the fuel cycle strategies and associated design activities: the case of Japan. J Nucl Mater 415:229–236
Wallenius J (2003) Neutronic aspects of inert matrix fuels for application in ADS. J Nucl Mater 320:142–146
Maschek W, Chen X, Delage F, Fernandez-Carretero A, Haas D, Matzerath Boccaccini C, Rineiski A, Smith P, Sobolev V, Thetford R, Wallenius J (2008) Accelerator driven systems for transmutation: fuel development, design and safety. Prog Nucl Energ 50:333–340
Guo T, Wang C, Lv J, Liang T (2016) Preparation of mesoporous zirconia microspheres as inert matrix. J Nucl Mater 481:66–72
Ye B, Miao JL, Li JL, Zhao ZC, Chang ZQ, Serra CA (2013) Fabrication of size-controlled CeO2 microparticles by a microfluidic sol–gel process as an analog preparation of ceramic nuclear fuel particles. J Nucl Mater 50:774–780
Wang L, Liang TX (2012) Ceramics for high level radioactive waste solidification. J Adv Ceram 1:194–203
Gao Y, Ma J, Zhao X, Hao S, Deng C, Liu B (2015) An improved internal gelation process for preparing ZrO2 ceramic microspheres without cooling the precursor solution. J Am Ceram Soc 98:2732–2737
De Almeida VF, Hunt RD, Collins JL (2010) Pneumatic drop-on-demand generation for production of metal oxide microspheres by internal gelation. J Nucl Mater 404:44–49
Kumar A, Radhakrishna J, Kumar N, Pai RV, Dehadrai JV, Deb AC, Mukerjee SK (2013) Studies on preparation of (U0.47,Pu0.53)O2 microspheres by internal gelation process. J Nucl Mater 434:162–169
Collins JL et al. (2013) Formulation and method for preparing gels comprising hydrous cerium oxide. U.S. Patent No. 8436052B2
Hunt RD, Collins JL, Johnson JA, Cowell BS (2017) Production of 75-150 mm and <75 mm of cerium dioxide microspheres in high yield and throughput using the internal gelation process. Ann Nucl Energ 105:116–120
Katalenich JA (2017) Production of cerium dioxide microspheres by an internal gelation sol–gel method. J Sol-Gel Sci Technol 82:654–663
Li X, Yang Y, Fu C, Huang Q, Sheng L, Chang Z, Serra CC (2014) A microfluidic-assisted fabrication of size-controlled porose CeO2 microspheres as an analog production of nuclear fuel beads Adv Sci Technol 94:55–68
Hunt RD, Collins JL, Cowell BS (2017) Use of boiled hexamethylenetetramine and urea to increase the porosity of cerium dioxide microspheres formed in the internal gelation process. J Nucl Mater 492:1–5
Khalil KMS, Elhamdy WA, Said AE-AA, Elsamahy AA (2016) Porous LaFeO3/Silica nanocomposites via sol-gel mixing involving citric acid. Colloid Surf A Physicochem Eng Asp 506:840–848
da Silva MFP, Matos JR, Isolani PC (2008) Synthesis, characterization and thermal analysis of 1:1 and 2:3 lanthanide(III) citrates. J Therm Anal Calorim 94:305–311
da Silva MFP, Carvalho FMd, Martins TS, Fantini MCA, Isolani PC (2010) The role of citrate precursors on the morphology of lanthanide oxide obtained by thermal decomposition. J Therm Anal Calorim 99:385–390
Spaulding L, Brittain HG (1983) Intermolecular energy transfer between lanthanide complexes.8. Tb(III) donor and Eu(III)acceptor complexes of citric acid. J Lumin 28:385–394
Popa M, Kakihana M (2001) Praseodymium oxide formation by thermal decomposition of a praseodymium complex. Solid State Ion 141-142:265–272
Carneiro J, Tedim J, Fernandes SCM, Freire CSR, Silvestre AJD, Gandini A, Ferreira MGS, Zheludkevich ML (2012) Chitosan-based self-healing protective coatings doped with cerium nitrate for corrosion protection of aluminum alloy 2024. Prog Org Coat 75:8–13
Kaliva M, Gabriel C, Raptopoulou CP, Terzis A, Salifoglou A (2008) pH-specific synthesis, isolation, spectroscopic and structural characterization of a new dimeric assembly of dinuclear vanadium(V)–citrate–peroxo species from aqueous solutions. Inorg Chim Acta 361:2631–2640
Vanhoylanda G, Pagnaera J, D’Haenb J, Mullensc S, Mullens J (2005) Characterization and structural study of lanthanum citrate trihydrate [La(C6H5O7)(H2O)2] ∙H2O. J Solid State Chem 178:166–171
Van Werde K, Mondelaers D, Vanhoyland G, Nelis D, Van Bael MK, Mullens J, Van Poucke LC (2002) Thermal decomposition of the ammonium zinc acetate citrate precursor for aqueous chemical solution deposition of ZnO. J Mater Sci 37:81–88
Sun X, Ma J, Chen X, Li Z, Deng C, Liu B (2018) Sol–gel preparation of ZrC–ZrO2 composite microspheres using fructose as a carbon source. J Sol-Gel Sci Techn 86:431–440
James GS (2005) Lange’s Handbook of Chemistry-Sixth Edition. McGraw-Hill, New York
Hao Z, Guo B, Liu H, Gan L, Xu Z, Chen L (2006) Synthesis of mesoporous silica using urea–formaldehyde resin as an active template. Micro Mesopor Mater 95:350–359
Hussein GAM (1996) Rare earth metal oxide: formation, characterization and catalytic activity thermoanalytical and applied pyrolysis review J Anal Appl Pyrol 37:111–149
Che P, Fang D, Zhang D, Feng J, Wang J, Hu N, Meng J (2005) Hydrothermal synthesis and crystal structure of a new two-dimensional zinc citrate complex. J Coord Chem 58:1581–1588
Acknowledgements
This work was financially supported by Key Program for International S&T Cooperation Projects of China (number 2016YFE0100700), Chinese National Natural Science Foundation (number 51420105006), and ‘The Thirteenth Five-Year Plan’’ Discipline Construction Foundation of Tsinghua University (number 2017HYYXKJS1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ding, X., Ma, J., Zhou, X. et al. Preparation of cerium dioxide microspheres by internal gelation with cerium citrate as precursor. J Sol-Gel Sci Technol 90, 296–304 (2019). https://doi.org/10.1007/s10971-019-04965-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-019-04965-w