Abstract
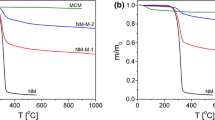
Mesoporous silica KIT-6 and MCM-41 with different pore sizes has been prepared through the sol–gel and hydrothermal method, respectively. The synthesized mesoporous materials were modified by a chemical modification using 3-aminopropyl triethoxysilane (APTES) to obtain KIT-6-NH2 and MCM-41-NH2 as drug delivery carriers. The mesostructure properties was fully characterized by scanning electron microscope (SEM), N2 sorption isotherm, Fourier transform infrared (FT-IR), X-ray diffraction (XRD). Loading of resveratrol (RSV) drug as a model into synthesized mesoporous carriers and amine functionalized forms were studied using thermogravimetric analysis (TGA) and UV–visible spectroscopy (UV–Vis). The loading uptake and release behavior of RSV was highly dependent on the textural properties (such as pore size and surface area) of mesoporous silica and modified carriers. The release of drugs was carefully studied in different pH. The effect of the synthesized carriers and RSV@mesoporous drug carriers on MCF-7 human breast cancer cell line viability was evaluated. Both carriers alone revealed no toxicity to MCF-7 cancer cells. But, RSV@carriers or RSV@modified carriers indicated an inhibition of cell livability, when compared to the non-encapsulated drug. RSV@modified mesoporous carriers demonstrated cell viability inhibition better than RSV@mesoporous carriers. The inhibition of cell viability of RSV@modified mesoporous carriers depends on surface area and functionalized groups of carriers more than pore size. First order, Higuchi, HixsoneCrowell and KorsmeyerePeppas release kinetic models were applied to the experimental data and the release was found to obey a first-order rate kinetic.

RSV@MCM-41-NH2 as a DDS caused the slow release of resveratrol, growing the bioavailability of the drug.
Highlights
-
The Resveratrol (RSV) is an experimental anticancer drug.
-
MCM-41 and KIT-6 were used as carriers for encapsulation of RSV drug.
-
The loading and release of RSV depends on surface area and pore size of carriers.
-
Amino groups play an important role in increased drug loading and release rate.
-
The effect of RSV@carriers was evaluated on MCF-7 human breast cancer.












Similar content being viewed by others
References
Wise DL (2000) Handbook of Pharmaceutical Controlled Release. Technology, 1st edn. Marcel Dekker Inc., New York
Brigger I, Dubernet C, Couvreur P (2002) Nanoparticles in cancer therapy and diagnosis. Adv Drug Deliv Rev 54:631–651
Chan S, Fauchet PM, Li Y, Rothberg LJ, Miller Bl (2000) Porous silicon microcavities for biosensing applications. Phys Status Solidi A 182:541–546
Dancil KPS, Greiner DP, Sailor MJ (1999) A porous silicon optical biosensor: detection of reversible binding of IgG to a protein A-modified surface. J Am Chem Soc 121:7925–7930
Coffer JL, Whitehead MA, Nagesha DK (2005) Porous silicon-based scaffolds for tissue engineering and other biomedical applications. Phys Status Solidi A 202:1451–1455
Canham LT, Reeves CL, Loni A (1997) Calcium phosphate nucleation on porous silicon: factors influencing kinetics in acellular simulated body fluids. Thin Sol Films 297:304–307
Canham LT, Reeves CL, King DO, Branfield PJ, Crabb JG, Ward MCL (1996) Bioactive polycrystalline silicon. Adv Mater 8:850–852
Anglin EJ, Schwartz MP, Ng VP, Perelman LA, Sailor MJ (2004) Engineering the chemistry and nanostructure of porous silicon Fabry–Pérot films for loading and release of a steroid. Langmuir 20:11264–11269
Charnay C, Begu S, Tourne-Peteilh C, Nicole L, Lerner DA, Devoisselle JM (2004) Inclusion of ibuprofen in mesoporous templated silica: drug loading and release property. Eur J Pharm Biopharm 57:533–540
Coffer JL, Montchamp JL, Aimone JB, Weis RP (2003) Routes to calcifled porous silicon: implications for drug delivery and biosensing. Phys Status Solidi A 197:336–339
Vaccari L, Canton D, Zaffaroni N, Villa R, Tormen M, di Fabrizio E (2006) Porous silicon as drug carrier for controlled delivery of doxorubicin anticancer agent. Microelectron Eng 83:1598–1601
Salonen J, Kaukonen AM, Hirvonen J, Lehto VP (2008) Mesoporous silicon in drug delivery applications. J Pharm Sci 97:632–653
Ayad MM, Salahuddin NA, Elnasr AA, Torad NL (2016) name functionalized mesoporous silica KIT-6 as a controlled release drug delivery. Microporous Mesoporous Mater 229:166–177
Slowing II, Vivero-Escoto JL, Wu CW, Lin VSY (2008) Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv Drug Deliv Rev 60:1278–1288
Zhang Q, Neoh KQ, Xu L, Lu S, Kang ET, Mahendran R, Chiong E (2014) Functionalized mesoporous silica nanoparticles with mucoadhesive and sustained drug release properties for potential bladder cancer therapy. Langmuir 30:6151–6161
Popat A, Hartono SB, Stahr F, Liu J, Qiao SZ, Lu G (2013) Mesoporous silica nanoparticles for bioadsorption, enzyme immobilization, and delivery carriers. Nanoscale 3:2801–2818
Sharma KK, Asefa T (2007) Efficient bifunctional nanocatalysts by simple post grafting of spatially-isolated catalytic groups on mesoporous materials. Angew Chem Int Ed 46:2879–2882
Regi MV, Ramila A, del Real RP, Perez-Pariente J (2001) A new property of MCM-41: drug delivery system. Chem Mater 13:308–311
Manzano M, Aina V, Arean CO, Balas F, Cauda V, Collila M, Delgado MR, Regi MV (2008) Studies on MCM-41 mesoporous silica for drug delivery: effect of particle morphology and amine functionalization. Chem Eng J 137:30–37
Bensacia N, Fechete I, Moulay S, Hulea O, Boos A, Garin F (2014) Kinetic and equilibrium studies of lead (II) adsorption from aqueous media by KIT-6 mesoporous silica functionalized with –COOH. Comptes Rendus Chim 17:869–880
Sanjini NS, Velmathi S (2013) Synthesis of gallium doped mesoporous KIT-6 for the photocatalytic degradation of dye. Asian J Chem 25:S69–S72
Zhao H, Liu S, Wang R, Zhang T (2015) Humidity-sensing properties of LiCl-loaded 3D cubic mesoporous silica KIT-6 composites. Mater Lett 147:54–57
Fazaeli R, Aliyan H, Foroushani SP, Mohagheghian Z, Heidari Z (2013) Mesoporous silica KIT-6 Heterogeneous catalysis Polyoxotungstate. Iran J Catal 3:129–137
Duan A, Li T, Zhao Z, Liu B, Zhou X, Jiang G, Liu J, Wei Y, Pan H (2015) Synthesis of hierarchically porous L-KIT-6 silica–alumina material and the super catalytic performances for hydrodesulfurization of benzothiophene. Appl Catal B Environ 165:763–773
Hu B, Liu H, Tao K, Xiong C, Zhou S (2013) Highly active doped mesoporous KIT-6 catalysts for metathesis of 1-butene and ethene to propene: the influence of neighboring environment of w species. J Phys Chem C 117:26385–26395
Kalepu S, Nekkanti V (2015) Insoluble drug delivery strategies: review of recent advances and business prospects. Acta Pharm Sin B 5:442–453
Kundu JK, Surh YJ (2008) Cancer chemopreventive and therapeutic potential of resveratrol: mechanistic perspectives. Cancer Lett 269:243–261
Jang MS, Cai EN, Udeani GO, Slowing KV, Thomas CF, Beecher CWW, Fong HSS, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM (1997) Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 275:218–220
Neves AR, Lúcio M, Lima JLC, Reis S (2012) Resveratrol in medicinal chemistry: a critical review of its pharmacokinetics, drug-delivery, and membrane interactions. Curr Med Chem 19:1663–1681
Stervbo U, Vang O, Bonnesen CA (2007) A reviews of the content of the putative chemopreventive phytoalexin resveratrol in red wine. Food Chem 101:449–457
Latifi L, Sohrabnezhad Sh, Hadavi M (2017) Mesoporous silica as a support for poorly soluble drug: influence of pH and amino group on the drug release. Microporous Mesoporous Mater 250:148–157
Wang W, Qi R, Shan W, Wang X, Jia Q, Zhao J, Zhang C, Ru H (2014) Synthesis of KIT-6 type mesoporous silicas with tunable pore sizes, wall thickness and particle sizes via the partitioned cooperative self-assembly process. Micro Mesoporus Mater 194:167–173
Moller K, Bein T (1998) Inclusion chemistry in periodic mesoporous hosts. Chem Mater 10:2950–2963
Zhu Y, Shi J, Li Y, Chen H, Shen W, Dong X (2005) Storage and release of ibuprofen drug molecules in hollow mesoporous silica spheres with modified pore surface. Microporous Mesoporous Mater 85:75–81
de Cássia da Silva R, Teixeira JA, Nunes WDG (2017) Resveratrol: a thermoanalytical study. Food Chem 237:561–565
Bruinsma PJ, Kim AY, Liu J, Baskaran S (1997) Mesoporous silica synthesized by solvent evaporation: spun fibers and spray-dried hollow spheres. Chem Mater 9:2507–2512
Sathish M, Viswanathan B, Viswanathan RP (2006) Alternate synthetic strategy for the preparation of CdS nanoparticles and its exploitation for water splitting. Int J Hydrog 31:891–898
Huh S, Chen HT, Wiench JW, Pruski M, Lin SY (2005) Cooperative catalysis by general acid and base bifunctionalized mesoporous silica nanospheres. Angew Chem Int Ed 44:1826–1830
Giraldo LF, Lopez BL, Perez L, Urrego S, Sierra L, Mesa M (2007) Mesoporous silica applications. Macromol Symp 258:129–141
Yang H, Feng Q (2010) Diorect synthesis of pore-expanded amino functionalized mesoporous silicas with dimethydecylamine and the effect expander dosage on their characterization and decolorization of sulphonated dyes. Micro Mesoporus Mater 135:124–130
Anbia M, Amirmahmoodi S (2011) Adsorption of phenolic compounds from aqueous solutions using functionalizedSBA-15 as a nano-sorbent. Sci Iran C 18:446–452
Billes F, Mohammed-Ziegler I, Mikosch H, Tyihák E (2007) Vibrational spectroscopy of resveratrol. Spectrochim Acta A 68:669–679
Gao L, Sun JH, Li YZ (2011) Functionalized bimodal mesoporous silicas as carriers for controlled aspirin delivery. J Solid State Chem 184:1909–1914
Gerweck LE, Seetharaman K (1996) Cellular pH gradient in tumor versus normal tissue: potential exploitation for the treatment of cancer. Cancer Res 56:1194–1198
Maria G, Berger D, Nastase S, Luta I (2012) Kinetic studies on the irinotecan release based on structural properties of functionalized mesoporous-silica supports. Microporous Mesoporous Mater 149:25–35
Popova MD, Szegedi A, Kolev IN, Mih_aly J, Tzankov BS, Momekov GT, Lambov NG, Yoncheva KP (2012) Carboxylic modified spherical mesoporous silicas аs drug delivery carriers. Int J Pharm 436:778–785
Acknowledgements
The authors are thankful to the post-graduate office of Guilan University for the support of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Latifi, L., Sohrabnezhad, S. Influence of pore size and surface area of mesoporous silica materilas (MCM-41 and KIT-6) on the drug loading and release. J Sol-Gel Sci Technol 87, 626–638 (2018). https://doi.org/10.1007/s10971-018-4742-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-018-4742-7