Abstract
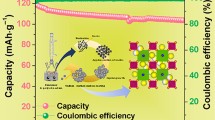
The solid solution series Na3V2-x Al x (PO4)2F3 (where x = 0, 0.02, 0.05, and 0.1) powders have been prepared using the Pechini method to study the effect of aluminum doping on the electrochemical properties of these cathode materials for lithium ion batteries. The structure, composition, and morphology of the compounds were investigated by X-ray diffraction, thermogravimetric and differential analysis, specific surface area, and pore size analysis using Brunauer-Emmett-Teller—Barret-Joyner-Halenda methods, scanning electron microscopy, elemental chemical analysis with induced coupled plasma-optical emission spectroscopy and charge/discharge galvanostatic experiments. X-ray diffraction indicates a tetragonal crystal structure for the Na3V2(PO4)2F3 phase, and all the Al compositions. The materials obtained have a microstructure consisting of nanoparticles (40–100 nm) with an average pore size 20 nm and 30 m2/g surface area. The phase with 0.05 moles of aluminum gave the best result electrochemical charge/discharge capacities of 123 to 101 mAh/g with cell voltage of 4.4 V vs. Li and a capacity retention of 82%, compared to undoped material which gave 128 to 63 mAh/g, and 49% capacity retention. Hence, Al-doped samples showed better electrochemical performance maybe attributable to improved structure stability compared to the undoped material. Those results suggest a promising material for lithium ion batteries.
Graphical Abstract









Similar content being viewed by others
References
Whittingham MS (2004) Lithium batteries and cathode materials. Chem Rev 104(10):4271–4302. doi:10.1021/cr020731c
Barker J, Saidi MY, Gover RKB, Burns P, Bryan A (2007) The effect of Al substitution on the lithium insertion properties of lithium vanadium fluorophosphate, LiVPO4F. J Power Sources 174(2):927–931. doi:10.1016/j.jpowsour.2007.06.079
Gover RKB, Bryan A, Burns P, Barker J (2006) The electrochemical insertion properties of sodium vanadium fluorophosphate, Na3V2(PO4)2F3. Solid State Ionics 177(17–18):1495–1500. doi:10.1016/j.ssi.2006.07.028
Ellis BL, Ramesh TN, Rowan-Weetaluktuk WN, Ryan DH, Nazar LF (2012) Solvothermal synthesis of electroactive lithium iron tavorites and structure of Li2FePO4F. J Mater Chem 22(11):4759–4766. doi:10.1039/C2JM15273H
Barker J, Saidi MY, Swoyer JL (2003) A sodium-ion cell based on the fluorophosphate compound NaVPO4 F. Electrochem Solid-State Lett 6(1):A1–A4. doi:10.1149/1.1523691
Wang J, Li X, Wang Z, Guo H, Li Y, He Z, Huang B (2013) Enhancement of electrochemical performance of Al-doped LiVPO4F using AlF3 as aluminum source. J Alloys Compd 581:836–842. doi:10.1016/j.jallcom.2013.07.147
Bai G, Yang Y, Shao H (2013) Synthesis and electrochemical properties of polyhedron-shaped Li3V2−xSnx(PO4)3 as cathode material for lithium-ion batteries. J Electroanal Chem 688:98–102. doi:10.1016/j.jelechem.2012.08.018
Liu Z-m, Wang X-y, Wang Y, Tang A-p, Yang S-y, He L-f (2008) Preparation of NaV1−xAlxPO4F cathode materials for application of sodium-ion battery. T Nonferr Metal Soc China 18(2):346–350. doi:10.1016/S1003-6326(08)60060-6
Song W, Ji X, Wu Z, Zhu Y, Li F, Yao Y, Banks CE (2014) Multifunctional dual Na3V2(PO4)2F3 cathode for both lithium-ion and sodium-ion batteries. RSC Adv 4(22):11375–11383. doi:10.1039/C3RA47878E
Zhang Q, Uchaker E, Candelaria SL, Cao G (2013) Nanomaterials for energy conversion and storage. Chem Soc Rev 42(7):3127–3171. doi:10.1039/C3CS00009E
Zhang B, Chen G, Liang Y, Xu P (2009) Structural and electrochemical properties of LiNi0.5Mn0.5 − xAlxO2 (x=0, 0.02, 0.05, 0.08, and 0.1) cathode materials for lithium-ion batteries. Solid State Ionics 180(4–5):398–404. doi:10.1016/j.ssi.2009.01.009
Liu H, Bi S, Wen G, Teng X, Gao P, Ni Z, Zhu Y, Zhang F (2012) Synthesis and electrochemical performance of Sn-doped Li3V2(PO4)3/C cathode material for lithium ion battery by microwave solid-state technique. J Alloys Compd 543:99–104. doi:10.1016/j.jallcom.2012.07.077
Liu H, Cao Q, Fu LJ, Li C, Wu YP, Wu HQ (2006) Doping effects of zinc on LiFePO4 cathode material for lithium ion batteries. Electrochem Commun 8(10):1553–1557. doi:10.1016/j.elecom.2006.07.014
Luo Y-z, He L-h, Liu X-h (2015) Effect of Mg doping on electrochemical performance of Li3V2(PO4)3/C cathode material for lithium ion batteries. T Nonferr Metal Soc China 25(7):2266–2271. doi:10.1016/S1003-6326(15)63840-7
Zhang B, Liu J-q, Zhang Q, Li Y-h (2010) Electrochemical performance of Al-substituted Li3V2(PO4)3 cathode materials synthesized by sol-gel method. T Nonferr Metals Soc China 20(4):619–623. doi:10.1016/S1003-6326(09)60188-6
Myung S-T, Kumagai N, Komaba S, Chung H-T (2001) Effects of Al doping on the microstructure of LiCoO2 cathode materials. Solid State Ionics 139(1–2):47–56. doi:10.1016/S0167-2738(00)00828-6
Lee KS, Bang HJ, Myung ST, Prakash J, Amine K, Sun YK (2007) Synthesis and electrochemical properties of spherical spinel Li1.05M0.05Mn1.9O4 (M = Mg and Al) as a cathode material for lithium-ion batteries by co-precipitation method. J Power Sources 174(2):726–729. doi:10.1016/j.jpowsour.2007.06.110
Zhong GB, Wang YY, Zhao XJ, Wang QS, Yu Y, Chen CH (2012) Structural, electrochemical and thermal stability investigations on LiNi0.5−xAl2xMn1.5−xO4 (0≤2x≤1.0) as 5V cathode materials. J Power Sources 216:368–375. doi:10.1016/j.jpowsour.2012.05.108
Acknowledgements
We would like to thank to SEP-CONACYT (CB-2012-01) for financial support through research project Ref. 189865, SEP-PROMEP, Ref. 103.5/12/7884 and the Universidad Autónoma de Nuevo León through PAICYT program. NPA also thanks CIMAV-Monterrey for the facilities to use some equipment for characterization of materials (A. Toxqui, A. Cavazos, L. De La Torre, L. Bautista, and F. E. Longoria for TGA-DTA, ICP-EOS, BET, FT-IR, and XRD measurements and assistance, respectively). Thanks to Dr Richard K.B. Gover for discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Pineda-Aguilar, N., Gallegos-Sánchez, V.J., Sánchez, E.M. et al. Aluminum doped Na3V2(PO4)2F3 via sol–gel Pechini method as a cathode material for lithium ion batteries. J Sol-Gel Sci Technol 83, 405–412 (2017). https://doi.org/10.1007/s10971-017-4398-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-017-4398-8