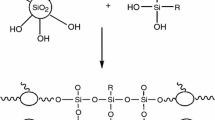
Abstract
In this study, the Taguchi method was used to design of experiment to obtain optimum conditions for hybrid Si–Zr sol–gel coating doped with cerium nitrate. The experimental design consisted of five different parameters at four levels. An orthogonal array of L16 type was utilized. With these experiences it was intended to find the suitable sol–gel deposition conditions for the aluminum alloy 6061. Chemical composition of the hybrid films were studied by attenuated total reflectance-fourier transform infrared spectroscopy. Field emission scanning electron microscopy was used to investigate the structural feature of coatings. Corrosion performance of coatings was evaluated by potentiodynamic polarization test. The response parameter of hybrid coatings was measured in terms of corrosion rate. The optimum processing parameters were predicted on the basis of data analysis and S/N ratio using analysis of variance. The results showed that by using optimal experimental parameters, the corrosion rate of aluminum alloy 6061 was decreased five orders of magnitude in compare to uncoated alloy 6061.
Graphical Abstract
This study has been based on design and optimizing effective parameters of hybrid organic-inorganic Si-Zr coatings doped with cerium reagent by Taguchi method. The effect of molar ratio of Si-bearing precursor, Zr content, Ce content, curing temperature and time was evaluated on corrosion rate of substrate. By deposition of the coatings on AA6061 the corrosion rate was decreased up to four orders of magnitude. Preparation of optimum coating led to decreasing in corrosion rate five orders of magnitude.









Similar content being viewed by others
Reference
Rosero-Navarro NC, Pellice SA, Duran A, Aparicio M (2008) Effects of Ce-containing sol–gel coatings reinforced with SiO2 nanoparticles on the protection of AA2024. Corros Sci 50:1283–1291
Osborne JH (2001) Observations on chromate conversion coatings from a sol–gel perspective. Prog Org Coat 41(4):280–286
Park RM, Bena JF, Stayner LT, Smith RJ, Gibb HJ, Lees PS (2004) Hexavalent chromium and lung cancer in the chromate industry: a quantitative risk assessment. J Risk Anal 24:1099–1108
Feng Z, Liu Y, Thompson GE, Skeldon P (2010) Sol–gel coatings for corrosion protection of 1050 aluminium alloy. Electrochim Acta 55:3518–3527
Gonzalez E, Pavez J, Azocar I, Zagal JH, Zhou X, Melo F, Thompson GE, Páez MA (2011) A silanol-based nanocomposite coating for protection of AA-2024 aluminium alloy. Electrochim Acta 56:7586–7595
Romano A, Fedel M, Deflorian F, Olivier MG (2011) Silane sol–gel film as pretreatment for improvement of barrier properties and filiform corrosion resistance of 6016 aluminium alloy covered by cataphoretic coating. Prog Org Coat 72:695–702
Wang D, Bierwagen GP (2009) Sol–gel coatings on metals for corrosion protection. Prog Org Coat 64:327–338
Zheludkevich ML, Salvado IM, Ferreira MGS (2005) Sol–gel coatings for corrosion protection of metals. J Mater Chem 15:5099–5111
Messaddeq SH, Pulcinelli SH, Santilli CV, Guastaldi AC, Messaddeq Y (1999) Microstructure and corrosion resistance of inorganic–organic (ZrO2–PMMA) hybrid coating on stainless steel. J Non-Cryst Solids 247:164–170
Sayilkan H, Sener S, Sener E, Sulu M (2003) The Sol–gel Synthesis and Application of Some Anticorrosion Coating Materials. Mater Sci Technol 39:733–740
Ono S, Tsuge H, Nishi Y, Hirano S (2004) Improvement of Corrosion Resistance of Metals by an Environmentally Friendly Silica Coating Method. J Sol–Gel Sci Technol 29:147–153
Sugama T (2005) Cerium acetate-modified aminopropylsilane triol: A precursor of corrosion-preventing coating for aluminum-finned condensers. J Coat Technol Res 2:649–659
Jianguo L, Gaoping G, Chuanwei Y (2006) Enhancement of the erosion–corrosion resistance of Dacromet with hybrid SiO2 sol–gel. Surf Coat Technol 200:4967–4975
Wu KH, Li MC, Chang TC, Yang CC (2006) Characterization and Corrosion Resistance of Organically Modified Silicate/MO2 (M = Zr, Ti, or Ce) Hybrid Coatings on a 6061-T6 Aluminum Alloy. J Polym Sci A Polym Chem 44:335–342
Rahimi H, Mozaffarinia R, Hojjati Najafabadi A (2013) Corrosion and Wear Resistance Characterization of Environmentally Friendly Solegel Hybrid Nanocomposite Coating on AA5083. J Mater Sci Technol 29(7):603–608
Rosario Elvira M, Alejandra Mazo M, Tamayo A, Rubio F, Rubio J, Oteo JL (2013) Study and characterization of organically modified Silica-Zirconia anti-graffiti coatings obtained by sol–gel. J Chem Chem Eng 7:120–131
Li L, He J, Lei J, Xu W, Jing X, Ou X, Wu S, Li N, Zhang S (2015) A sol–bath–gel approach to prepare hybrid coating for corrosion protection of aluminum alloy. Surf Coat Technol 279:72–78
Bahrami M, Borhani GhH, Bakhshi SR, Ghasemi A (2015) Preparation and evaluation of corrosion behavior of GPTMS-TEOS hybrid coatings containing Zr and Ce on aluminum alloy 6061-T6. J Sol–gel Sci Technol 76:552–561
Byrne D.M, Taguchi S (1986) The Taguchi approach to parameter design, vol 40. 40th annual quality congress, Anaheim, pp 168–177
Antony J, Antony FJ (2001) Teaching the Taguchi method to industrial engineers. Work Study 50(4):141–149
Boerio FJ (1983) Infrared spectra of polymers and coupling agents adsorbed onto oxidize aluminium. Polym Prepr 22:297–304
Gabrielli L, Russo L et al. (2013) Epoxide Opening versus Silica Condensation during Sol–Gel Hybrid Biomaterial Synthesis. Chem Eur J 19:7856–7864
Tiwari SK, Adhikary J, Singh TB, Singh R (2009) Preparation and characterization of sol–gel derived yttria doped zirconia coatings on AISI 316L. Thin Solid Films 517:4502–4508
Bell RJ, Dean P (1966) Properties of Vitreous Silica: Analysis of Random Network Models. Nature 212:1354–1356
Barglik-Chory C, Schubert U (1995) Organically substituted titanium alkoxides with unsaturated organic groups. J Sol–Gel Sci Technol 5:135–142
Ebrahim Amani M, Bandosz Teresa J (2014) Carbon Coated Silica Doped With Cerium/Zirconium Mixed Oxides as NO2 Adsorbent at Ambient Conditions. J Phys Chem C 118:8982–8992
Gotze J, Mockel R, Langhof N, Hengst M, Klinger M (2008) Silicification of Wood in the Laboratory. Ceramics 52(4):268–277
Feng Z, Liu Y, Thompson GE, Skeldon P (2010) Crack-free sol–gel coatings for protection of AA1050 aluminium alloy. Surf Interface Anal 42:306–310
Beuth JL (1992) Cracking of thin bonded films in residual tension. Int J Solids Struct 29:1657–1675
Liu Y, Feng Z, Walton J, Thompson GE, Skeldon P, Zhou X (2013) Comparison of the behaviors of chromate and sol–gel coatings on aluminium. Surf Interface Anal 45:1446–1451
Samer Darwich (2012) Corrosion protection concepts for aluminium and magnesium alloy coated with silica films prepared by water based sol–gel process. PhD Thesis, Technischen Universitat Chemnitz, Germany.
Bendell A, Disney J, Pridmore WA (1989) Taguchi methods: applications in world industry. IFS Publications, UK
Franquet A, Le Pen C, Terryn H, Vereecken J (2003) Effect of bath concentration and curing time on the structure of nonfunctional thin organosilane layers on aluminium. Electrochim Acta 48(9):1245–1255
Phanasgaonkar A, Raja VS (2009) Influence of curing temperature, silica nanoparticles- and cerium on surface morphology and corrosion behaviour of hybrid silane coatings on mild steel. Surf Coat Technol 203:2260–2271
Kaladhar M, Subbaiah KV, Srinivasa Rao C, Narayana Rao K (2011) Application of Taguchi approach and utility concept in solving the multi-objective problem when turning AISI 202 austenitic stainless steel. J Eng Sci Technol Rev 4(1):55–61
Tiwari A, Hihara LH (2007) High silicone content barrier coatings for corrosion protection of metals. TRI-service corrosion conference, Denver, 3–7 December
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Bahrami, M., Borhani, G.H., Bakhshi, S.R. et al. Optimization of effective processing parameters of hybrid anti-corrosion Si/Zr sol–gel coatings doped with cerium salt for aluminum alloy 6061. J Sol-Gel Sci Technol 81, 921–933 (2017). https://doi.org/10.1007/s10971-016-4255-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-016-4255-1