Abstract
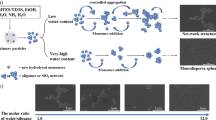
Silica micro spheres could be obtained through a semibatch sol–gel process, which was carried out by adding a solution of tetraethyl orthosilicate in ethanol into a mixture batch of KCl, ethanol, NH3•H2O, and water. Compared with other methods the semibatch sol–gel method could produce micro size silica spheres with narrow size distribution. The influence of reaction factors to the size and size distribution of silica spheres were studied. K+ could help obtaining large size silica but also could reduce the charge shielding effect, which caused the collision of silica spheres. Water and NH3•H2O were necessary for the hydrolysis of tetraethyl orthosilicate. However, too much water could form thicker hydration shell around K+, and more NH3•H2O in mixture batch would increase number of the nuclei of the solution. When the consumption rate of active silanol for the growing of silica could not offset its formation rate, a small silica size, and broad size distribution would occour. Therefore, the amount of tetraethyl orthosilicate in the reaction and its adding rate should be careful controlled. It was also found that the reaction temperature and holding time, which is the time for stirring of solution after tetraethyl orthosilicate injection, had no significant influence on the particle size.
Graphical Abstract








Similar content being viewed by others
References
Zhao JJ, Duan YY, Wang XD, Zhang XR, Han YH, Gao YB, Lv ZH, Yu HT, Wang BX (2013) Optical and radiative properties of infrared opacifier particles loaded in silica aerogels for high temperature thermal insulation. Int J Therm Sci 70:54–64
Yoshioka S, Takeoka Y (2014) Production of colourful pigments consisting of amorphous arrays of silica particles. Chem Phys Chem 15:2209–2215
Rajanna SK, Kumar D, Vinjamur M, Mukhopadhyay M (2015) Silica aerogel microparticles from rice husk ash for drug delivery. Ind Eng Chem Res 54:949–956
Sun J, Cui X, Zhang C, Zhang C, Ding R, Xu Y (2015) A broadband antireflective coating based on a double-layer system containing mesoporous silica and nanoporous silica. J Mater Chem C 3:7187–7194
Jaroenworaluck A, Sunsaneeyametha W, Kosachan N, Stevens R (2006) Characteristics of silica-coated TiO2 and its UV absorption for sunscreen cosmetic applications. Surf Interface Anal 38:473–477
Hayes R, Ahmed A, Edge T, Zhang H (2014) Core-shell particles: preparation, fundamentals and applications in high performance liquid chromatography. J Chromatogr A 1357:36–52
Eriksson K, Göthelid E, Puglia C, Bäckvall J-E, Oscarsson S (2013) Performance of a biomimetic oxidation catalyst immobilized on silica particles. J Catal 303:16–21
Thomas ELH, Nelson GW, Mandal S, Foord JS, Williams OA (2014) Chemical mechanical polishing of thin film diamond. Carbon 68:473–479
D’Acunzi M, Mammen L, Singh M, Deng X, Roth M, Auernhammer GK, Butt HJ, Vollmer D (2010) Superhydrophobic surfaces by hybrid raspberry-like particles. Faraday Discuss 146:35–48
Hoefnagels HF, Wu D, de With G, Ming W (2007) Biomimetic superhydrophobic and highly oleophobic cotton textiles. Langmuir 23:13158–13163
Wang H, Fang J, Cheng T, Ding J, Qu L, Dai L, Wang X, Lin T (2008) One-step coating of fluoro-containing silica nanoparticles for universal generation of surface superhydrophobicity. Chem Commun 7:877–879
Xue CH, Jia S-T, Zhang J, Tian LQ (2009) Superhydrophobic surfaces on cotton textiles by complex coating of silica nanoparticles and hydrophobization. Thin Solid Films 517:4593–4598
Ide M, Wallaert E, Van Driessche I, Lynen F, Sandra P, Van Der Voort P (2011) Spherical mesoorous silica particles by spray drying: doubling the retention factor of HPLC columns. Microporous Mesoporous Mat 142:282–291
He JL, Chen XH, Zhu WJ, Ma WH, Xiao YY, Li J, Zhang H (2014) Preparation of porous silica microspheres by polymerization-induced colloid aggregation method. Adv Mat Res 898:132–135
Meng Z, Xue C, Zhang Q, Yu X, Xi K, Jia X (2009) Preparation of highly monodisperse hybrid silica nanospheres using a one-step emulsion reaction in aqueous solution. Langmuir 25:7879–7883
Chang SM, Lee M, Kim WS (2005) Preparation of large monodispersed spherical silica particles using seed particle growth. J Colloid Interface Sci 286:536–542
Chen SL, Yuan G, Hu CT (2011) Preparation and size determination of monodisperse silica microspheres for particle size certified reference materials. Powder Technol 207:232–237
Stöber W, Fink A, Bohn E (1968) Controlled growth of monodisperse silica spheres in the micron size range. J Colloid Interface Sci 26:62–69
Chen SL, Dong P, Yang GH, Yang JJ (1996) Characteristic aspects of formation of new particles during the growth of monosize silica seeds. J Colloid Interface Sci 180:237–241
Giesche H (1994) Synthesis of monodispersed silica powders II. Controlled growth reaction and continuous production process. J Eur Ceram Soc 14:205–214
Nozawa K, Gailhanou H, Raison L, Panizza P, Ushiki H, Sellier E, Delville JP, Delville MH (2005) Smart control of monodisperse Stober silica particles: effect of reactant addition rate on growth process. Langmuir 21:1516–1523
Nagao D, Satoh T, Konno M (2000) A generalized model for describing particle formation in the synthesis of monodisperse oxide particles based on the hydrolysis and condensation of tetraethyl orthosilicate. J Colloid Interface Sci 232:102–110
Matsoukas T, Gulari E (1989) Monomer-addition growth with a slow initiation step: A growth model for silica particles from alkoxides. J Colloid Interface Sci 132:13–21
Nakabayashi H, Yamada A, Noba M, Kobayashi Y, Konno M, Nagao D (2010) Electrolyte-added one-pot synthesis for producing monodisperse, micrometer-sized silica particles up to 7 microm. Langmuir 26:7512–7515
Matsoukas T, Gulari E (1988) Dynamics of growth of silica particles from ammonia-catalyzed hydrolysis of tetra-ethyl-orthosilicate. J Colloid Interface Sci 124:252–261
Matsuoka J, Numaguchi M, Yoshida S, Soga N (2000) Heat of reaction of the hydrolysis-polymerization process of tetraethyl orthosilicate in acidic condition. J Sol-Gel Sci Technol 19:661–664
Rimer JD, Trofymluk O, Lobo RF, Navrotsky A, Vlachos DG (2008) Thermodynamics of silica nanoparticle self-assembly in basic solutions of monovalent cations. J Phys Chem C 112:14754–14761
Acknowledgments
This work is financial supported by the National Natural Science Foundation of China (Grant No. 51203065), and the Fundamental Research Funds for the Central Universities (Grant No. JUSRP51622A).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Luo, X., Dong, J., Zhang, L. et al. Preparation of silica micro spheres via a semibatch sol–gel method. J Sol-Gel Sci Technol 81, 669–677 (2017). https://doi.org/10.1007/s10971-016-4245-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-016-4245-3