Abstract
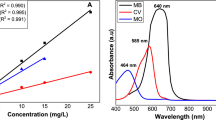
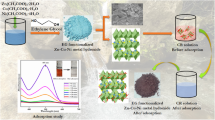
In this study, Zinc Oxide (ZnO)–Cadmium Oxide (CdO) nanocomposite has been synthesized by reverse microemulsion method and used as adsorbent to remove methyl blue from aqueous solution. The synthesized products were studied by X-ray diffraction, Brunauer–Emmett–Teller surface area analysis, thermogravimetric and differential thermal analysis and transmission electron microscopy. The effect of parameters, including adsorbent dosage, contact time, methyl blue concentration and ZnO/CdO weight ratio on the adsorption properties were investigated. The results showed that the ZnO–CdO composites have a nearly spherical shape with a size of tens of nanometers and exhibit the surface area of 9.5 m2/g. The synthesized products showed excellent performance for fast and efficient removal of dye contaminant, methyl blue, in aqueous solution.
Graphical Abstract








Similar content being viewed by others
References
World Health Organization (2007) p 1, ISBN:9789241595223
Bailey SE, Olin TJ, Bricka RM, Adrian DD (1999) A review of potentially low-cost sorbents for heavy metals. Water Res 33:2469–2479
Rostamian R, Najafi M, Rafati AA (2011) Synthesis and characterization of thiol-functionalized silica nano hollow sphere as a novel adsorbent for removal of poisonous heavy metal ions from water: kinetics, isotherms and error analysis. Chem Eng J 171:1004–1011
Yuan L, Liu Y (2013) Removal of Pb(II) and Zn(II) from aqueous solution by ceramisite prepared by sintering bentonite, iron powder and activated carbon. Chem Eng J 215–216:432–439
Vaghela SS, Jethva AD, Mehta BB, Dave SP, Adimurthy S, Ramachandraiah G (2005) Laboratory studies of electrochemical treatment of industrial azo dye effluent. Environ Sci Technol 39:2848–2855
Hunsom M, Pruksathorn K, Damronglerd S, Vergnes H, Duverneuil P (2005) Electrochemical treatment of heavy metals (Cu2+, Cr6+, Ni2+) from industrial effluent and modeling of copper reduction. Water Res 39:610–616
Matlock MM, Howerton BS, Atwood DA (2002) Chemical precipitation of heavy metals from acid mine drainage. Water Res 36:4757–4764
Altın İ, Sökmen M, Bıyıklıoğlu Z (2016) Sol gel synthesis of cobalt doped TiO2 and its dye sensitization for efficient pollutant removal. Mater Sci Semicond Process 45:36–44
Maturana HA, Peric IM, Rivas BL, Pooley SA (2011) Interaction of heavy metal ions with an ion exchange resin obtained from a natural polyelectrolyte. Polym Bull 67:669–676
Juang RS, Huang HL (2003) Mechanistic analysis of solvent extraction of heavy metals in membrane contactors. J Membr Sci 213:125–135
Muthukrishnan M, Guha BK (2006) Heavy metal separation by using surface modified nanofiltration membrane. Desalination. 200:351–353
Li X, Zhu Z, Zhao Q, Wang L (2011) Photocatalytic degradation of gaseous toluene over ZnAl2O4 prepared by different methods: a comparative study. J Hazard Mater 186:2089–2096
Volikov AB, Ponomarenko SA, Konstantinov AI, Hatfield K, Perminova IV (2016) Nature-like solution for removal of direct brown 1 azo dye from aqueous phase using humics-modified silica gel. Chemosphere 145:83–88
Motlagh MM, Hassanzadeh-Tabrizi SA, Saffar-Teluri A (2015) Sol–gel synthesis of Mn2O3/Al2O3/SiO2 hybrid nanocomposite and application for removal oforganic dye. J Sol–Gel Sci Technol 73:9–13
Yousef A, Barakat Nasser AM, Amna T, Unnithan AR, Al-Deyab SS, Kim HY (2012) Influence of CdO-doping on the photoluminescence properties of ZnO nanofibers: effective visible light photocatalyst for waste water treatment. J Lumin 132:1668–1677
Mosquera E, Pozo I, Morel M (2013) Structure and red shift of optical band gap in CdO–ZnO nanocomposite synthesized by the sol gel method. J Solid State Chem 206:265–271
Karunakaran C, SakthiRaadha S, Gomathisankar P, Vinayagamoorthy P (2013) Hydrothermal and sonochemical preparation and photocatalytic and bactericidal activities of ZnFe2O4–SnO2 nanocomposite. Superlattices Microstruct 60:487–499
Alizadeh S, Hassanzadeh-Tabrizi SA (2015) MoO3 fibers and belts: molten salt synthesis, characterization and optical properties. Ceram Int 41:10839–10843
Foletto EL, Battiston S, Simoes JM, Bassaco MM, Pereira LSF, Flores EMM, Muller EI (2012) Synthesis of ZnAl2O4 nanoparticles by different routes and the effect of its pore size on the photocatalytic process. Microporous Mesoporous Mater 163:29–33
Hassanzadeh-Tabrizi SA, Taheri-Nassaj E (2010) Compressibility and sinterability of Al2O3–YAG nanocomposite powder synthesized by an aqueous sol–gel method. J Alloys Compd 506:640–644
Tajizadegan H, Jafari M, Rashidzadeh M, Saffar-Teluri A (2013) A high activityadsorbent of ZnO–Al2O3 nanocomposite particles: synthesis, characterizationand dye removal efficiency. Appl Surf Sci 276:317–322
Shojaei S, Hassanzadeh-Tabrizi SA, Ghashang M (2014) Reverse microemulsion synthesis and characterization of CaSnO3 nanoparticles. Ceram Int 40:9609–9613
Pournajaf R, Hassanzadeh-Tabrizi SA, Ghashang M (2014) Effect of surfactants on the synthesis of Al2O3–CeO2 nanocomposite using a reverse microemulsion method. Ceram Int 40:4933–4937
Tavakoli H, Sarraf-Mamoory R, Zarei AR (2015) Solvothermal synthesis of copper nanoparticles loaded on multi-wall carbon nanotubes as catalyst for thermal decomposition of ammonium perchlorate. J Adv Mater Process 3:3–10
Sing SW, Everett DH, Haul RAW, Moscou L, Pierotti RA, Rouquerol J, Siemieniewska T (1985) Reporting physisorption data for gas/solid systems, with special reference to the determination of surface area and porosity. Pure Appl Chem 57:603
An C, Wang Y, Huang Y, Xu Y, Jiao L, Yuan H (2014) Porous NiCo2O4 nanostructures for high performance supercapacitors via a microemulsion technique. Nano Energy 10:125–134
Choi J, Kim J, Yoo KS, Lee TG (2007) Synthesis of mesoporous TiO2/γ–Al2O3 composite granules with different sol composition and calcination temperature. Powder Technol 181:83–88
Wani IA, Khatoon S, Ganguly A, Ahmed J, Ahmad T (2013) Structural characterization and antimicrobial properties of silver nanoparticles prepared by inverse microemulsion method. Colloids Surf B 101:243–250
Hassanzadeh-Tabrizi SA, Hosseini Badr H, Alizadeh S (2016) In situ synthesis of vanadium carbide–copper nanocomposite by a modified mechanochemical combustion method. Ceram Int 42:9371–9374
Michael Hu ZC, Michael Harris T, Charles Byers H (1998) Nucleation and growth for synthesis of nanometric zirconia particles by forced hydrolysis. J Colloid Interface Sci 198:87–99
Vidyasagar CC, Naik YA, Venkatesh TG, Viswanatha R (2011) Solid-state synthesis and effect of temperature on optical properties of Cu–ZnO, Cu–CdO and CuO nanoparticles. Powder Technol 214:337–343
Fan L, Luo C, Li X, Lu F, Qiu H, Sun M (2012) Fabrication of novel magnetic chitosan grafted with graphene oxide to enhance adsorption properties for methyl blue. J Hazard Mater 215–216:272–279
Crini G, Gimbert F, Robert C, Martel B, Adam O, Morin-Crini N, et al. (2008) The removal of basic blue 3 from aqueous solutions by chitosan-based adsorbent: batch studies. J Hazard Mater 153:96–106
Yavari S, Mahmodi NM, Teymouri P, Shahmoradi B, Maleki A (2016) Cobalt ferrite nanoparticles: preparation, characterization and anionic dye removal capability. J Taiwan Inst Chem Eng 59:320–329
Ni ZM, Xia SJ, Wang LG, Xing FF, Pan GX (2007) Treatment of methyl orange by calcined layered double hydroxides in aqueous solution: adsorption property and kinetic studies. J Colloid Interface Sci 316:284–291
Hassanzadeh-Tabrizi SA, Motlagh MM, Salahshour S (2016) Synthesis of ZnO/CuO nanocomposite immobilized on γ-Al2O3 and application for removal of methyl orange. Appl Surf Sci 384:237–243
Haldorai Yuvaraj, Shim Jae-Jin (2014) An efficient removal of methyl orange dye from aqueous solution by adsorption onto chitosan/MgO composite: a novel reusable adsorbent. Appl Surf Sci 292:447–453
Pandiselvi Kannusamy, Thambidurai Sivalingam (2013) Synthesis of porous chitosan–polyaniline/ZnO hybrid composite and application for removal of reactive orange 16 dye. Colloids Surf B Biointerfaces 108:229–238
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Rights and permissions
About this article
Cite this article
Khalili, E., Hassanzadeh-Tabrizi, S. ZnO–CdO nanocomposite: microemulsion synthesis and dye removal ability. J Sol-Gel Sci Technol 81, 475–482 (2017). https://doi.org/10.1007/s10971-016-4211-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-016-4211-0