Abstract
A novel graphene aerogel (GA)/ferric oxide (Fe2O3)/ammonium perchlorate (AP) nanostructured energetic composite was prepared by a facile sol–gel method and supercritical carbon dioxide drying technique. In this study, the morphology and structure of the obtained GA/Fe2O3/AP nanostructured energetic composite were characterized by scanning electron microscopy, nitrogen sorption tests and X-ray diffraction. The thermal decomposition characteristic was investigated by thermogravimetry and differential scanning calorimetry. The results demonstrated that Fe2O3 and AP dispersed in the as-prepared energetic composite at nanometer, showing promising catalytic effects for the thermal decomposition of AP. For the nanostructured energetic composite, GA and Fe2O3 played a catalytic role in the thermal decomposition of AP. Only one decomposition step was observed, instead of two, which was common in previous report. The decomposition temperature of the nanocomposite was obviously decreased as well. Moreover, the total heat release increased significantly. The experimental results showed that the as-prepared GA/Fe2O3/AP nanostructured energetic composite could be a promising candidate material for the solid propellants.
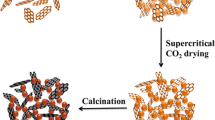
Graphical Abstract
A novel GA/Fe2O3/AP nanostructured energetic composite was prepared by a facile sol–gel method and supercritical carbon dioxide drying technique. Fe2O3 and AP nanoparticles are added and trapped in the porous three-dimensional networks of GA. The decomposition temperature of the nanocomposite was obviously decreased, and the total heat release increased significantly. Moreover, the thermal decomposition mechanism of the nanocomposite was analyzed.









Similar content being viewed by others
References
Rossi C, Zhang K, Esteve D, Alphonse P, Tailhades P, Vahlas C (2007) Nanoenergetic materials for MEMS: a review. Microelectromech Syst 16(4):919–931
Ahn JY, Kim WD, Cho K, Lee D, Kim SH (2011) Effect of metal oxide nanostructures on the explosive property of metastable intermolecular composite particles. Powder Technol 211(1):65–71
Miller HA, Kusel BS, Danielson ST, Neat JW, Avjian EK, Pierson SN, Budy SM, Ball DW, Iacono ST, Kettwich SC (2013) Metastable nanostructured metallized fluoropolymer composites for energetics. J Mater Chem A 1(24):7050–7058
Asay BW, Son SF, Busse JR, Oschwald DM (2004) Ignition characteristics of metastable intermolecular composites. Propellants Explos Pyrotech 29(4):216–219
Prakash A, McCormick AV, Zachariah MR (2005) Synthesis and reactivity of a super-reactive metastable intermolecular composite formulation of Al/KMnO4. Adv Mater 17(7):900–903
Thiruvengadathan R, Bezmelnitsyn A, Apperson S, Staley C, Redner P, Balas W, Nicolich S, Kapoor D, Gangopadhyay K, Gangopadhyay S (2011) Combustion characteristics of novel hybrid nanoenergetic formulations. Combust Flame 158(5):964–978
Shen J, Qiao Z, Zhang K, Wang J, Li R, Xu H, Yang G, Nie F (2014) Effects of nano-Ag on the combustion process of Al–CuO metastable intermolecular composite. Appl Therm Eng 62(2):732–737
Tillotson T, Gash A, Simpson R, Hrubesh L, Satcher J Jr, Poco J (2001) Nanostructured energetic materials using sol–gel methodologies. J Non-Cryst Solids 285(1):338–345
Prentice D, Pantoya ML, Gash AE (2006) Combustion wave speeds of sol–gel-synthesized tungsten trioxide and nano-aluminum: the effect of impurities on flame propagation. Energy Fuels 20(6):2370–2376
Leventis N, Chandrasekaran N, Sadekar AG, Sotiriou-Leventis C, Lu H (2009) One-pot synthesis of interpenetrating inorganic/organic networks of CuO/resorcinol–formaldehyde aerogels: nanostructured energetic materials. J Am Chem Soc 131(13):4576–4577
Zhang J, Yang GC, Nie FD (2010) Preparation of RDX/RF nanocomposite energetic particles by emulsion–sol–gel technique. Chin J Energ Mater 18(6):643–647
Wang X, Li J, Luo Y, Huang M (2014) A novel ammonium perchlorate/graphene aerogel nanostructured energetic composite: preparation and thermal decomposition. Sci Adv Mater 6(3):530–537
Gao K, Li G, Luo Y, Wang L, Shen L, Wang G (2014) Preparation and characterization of the AP/Al/Fe2O3 ternary nano-thermites. J Therm Anal Calorim 118(1):43–49
Worsley MA, Pauzauskie PJ, Olson TY, Biener J, Satcher JH Jr, Baumann TF (2010) Synthesis of graphene aerogel with high electrical conductivity. J Am Chem Soc 132(40):14067–14069
Zhang X, Sui Z, Xu B, Yue S, Luo Y, Zhan W, Liu B (2011) Mechanically strong and highly conductive graphene aerogel and its use as electrodes for electrochemical power sources. J Mater Chem 21(18):6494–6497
Chen W, Yan L (2011) In situ self-assembly of mild chemical reduction graphene for three-dimensional architectures. Nanoscale 3(8):3132–3137
Shusser MC, Culick FE, Cohen NS (2002) Combustion response of ammonium perchlorate composite propellants. J Propuls Power 18(5):1093–1100
Ma Z, Li F, Bai H (2006) Effect of Fe2O3 in Fe2O3/AP composite particles on thermal decomposition of AP and on burning rate of the composite propellant. Propellants Explos Pyrotech 31(6):447–451
Han X, Sun Y, Wang T, Lin ZK, Li SF, Zhao F, Liu Z, Yi J, Ren X (2008) Thermal decomposition of ammonium perchlorate based mixture with fullerenes. J Therm Anal Calorim 91(2):551–557
Reshmi S, Catherine KB, Nair CR (2011) Effect of carbon nanotube on the thermal decomposition characteristics of selected propellant binders and oxidisers. Int J Nanotechnol 8(10):979–987
Yuan Y, Jiang W, Wang Y, Shen P, Li F, Li P, Zhao F, Gao H (2014) Hydrothermal preparation of Fe2O3/graphene nanocomposite and its enhanced catalytic activity on the thermal decomposition of ammonium perchlorate. Appl Surf Sci 303:354–359
Li N, Geng Z, Cao M, Ren L, Zhao X, Liu B, Tian Y, Hu C (2013) Well-dispersed ultrafine Mn3O4 nanoparticles on graphene as a promising catalyst for the thermal decomposition of ammonium perchlorate. Carbon 54:124–132
Hummers WS Jr, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80(6):1339
Yang S, Feng X, Müllen K (2011) Sandwich-like, graphene-based titania nanosheets with high surface area for fast lithium storage. Adv Mater 23(31):3575–3579
Ma Z, Wu R, Song J, Li C, Chen R, Zhang L (2012) Preparation and characterization of Fe2O3/ammonium perchlorate (AP) nanocomposites through ceramic membrane anti-solvent crystallization. Propellants Explos Pyrotech 37(2):183–190
Li N, Cao M, Wu Q, Hu C (2012) A facile one-step method to produce Ni/graphene nanocomposites and their application to the thermal decomposition of ammonium perchlorate. CrystEngComm 14(2):428–434
Zhang Y, Liu X, Nie J, Yu L, Zhong Y, Huang C (2011) Improve the catalytic activity of α-Fe2O3 particles in decomposition of ammonium perchlorate by coating amorphous carbon on their surface. J Solid State Chem 184(2):387–390
Chaturvedi S, Dave PN (2013) A review on the use of nanometals as catalysts for the thermal decomposition of ammonium perchlorate. J Saudi Chem Soc 17(2):135–149
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lan, Y., Jin, M. & Luo, Y. Preparation and characterization of graphene aerogel/Fe2O3/ammonium perchlorate nanostructured energetic composite. J Sol-Gel Sci Technol 74, 161–167 (2015). https://doi.org/10.1007/s10971-014-3590-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-014-3590-3