Abstract
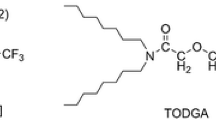
The study of the structure and properties of extractants is of great significance for developing new extractants. For uranium extraction and recovery requirements in spent fuel reprocessing, three kinds of extractants with different ether-oxygen chains, N,N,N’N’-tetraoctyl-3-oxapentanediamide (TODGA), N,N,N’N’-tetraoctyl-3,6-dioxaoctanediamide (TODOODA) and N,N,N’N’-tetraoctyl-3,6,9-trioxaundecanediamide (TOUDA) were synthesized to study the influence of the ether-oxygen chain skeleton on the extraction of uranium from nitrate media. TODOODA shows a good extraction ability for uranyl ions at high nitric acid concentrations. Equimolar series method, FT–IR spectrum and MALDI–TOF–MS spectra indicated that the extracted structure was formed as [UO2NO3·TODGA]NO3, [UO2NO3·TODOODA]NO3 and [UO2NO3·TOUDA]NO3.








Similar content being viewed by others
References
Schiermeier Q, Tollefson J, Scully T, Witze A, Morton O (2008) Energy alternatives: electricity without carbon. Nature 454:816–823. https://doi.org/10.1038/454816a
Megía PJ, Vizcaíno AJ, Calles JA, Carrero A (2021) Hydrogen production technologies: from fossil fuels toward renewable sources. A mini review. Energy Fuels 35:16403–16415. https://doi.org/10.1021/acs.energyfuels.1c02501
Wang H, Cui T, Sui J, Mocilac P, Wang Y, Guo Z (2022) Efficient UO22+ extraction by DAPhens with asymmetric terminal groups: the molecular design, spectral titration, liquid-liquid extraction and mechanism study. Sep Purif Technol 282:120046. https://doi.org/10.1016/j.seppur.2021.120046
Tang L, Ren S, Zhang T, Wei X, Li M, Yin X, Wei S (2021) UO22+-imprinted thermoresponsive hydrogel for accumulation of uranium from seawater. Chem Eng J 425:130589. https://doi.org/10.1016/j.cej.2021.130589
Garg N, Rastogi L, Bera S, Ballal A, Balramkrishna MV (2022) ArsenazoIII functionalized gold nanoparticles: SPR based optical sensor for determination of uranyl ions (UO22+) in groundwater. Green Anal Chem 3:100032. https://doi.org/10.1016/j.greeac.2022.100032
Gul UD, Senol ZM, Gursoy N, Simsek S (2019) Effective UO22+ removal from aqueous solutions using lichen biomass as a natural and low-cost biosorbent. J Environ Radioact 205–206:93–100. https://doi.org/10.1016/j.jenvrad.2019.05.008
Bae SY, Southard GL, Murray GM (1999) Molecularly imprinted ion exchange resin for purification, preconcentration and determination of UO22+ by spectrophotometry and plasma spectrometry. Anal Chim Acta 397:173–181. https://doi.org/10.1016/S0003-2670(99)00402-X
Vats BG, Bhattacharyya A, Sanyal K, Kumar M, Gamare JS, Kannan S (2021) Piperazinyl-based diamide ligand for selective precipitation of actinyl (UO22+/PuO22+) Ions with fast kinetics. Inorg Chem 60:17529–17536. https://doi.org/10.1021/acs.inorgchem.1c02056
Sun T, Xu C, Fu J, Chen Q, Chen J, Shen X (2017) Extraction of U(VI) by the ionic liquid hexyltributylphosphonium bis(trifluoromethylsulfonyl)imides: an experimental and theoretical study. Sep Purif Technol 188:386–393. https://doi.org/10.1016/j.seppur.2017.07.055
Paramanik M, Panja S, Dhami PS, Yadav JS, Kaushik CP, Ghosh SK (2018) Unique reversibility in extraction mechanism of U compared to solvent extraction for sorption of U(VI) and Pu(IV) by a novel solvent impregnated resin containing trialkyl phosphine oxide functionalized ionic liquid. J Hazard Mater 354:125–132. https://doi.org/10.1016/j.jhazmat.2018.05.003
Chandrasekar A, Suresh A, Joshi M, Sundararajan M, Ghanty TK, Sivaraman N (2019) Highly selective separations of U(VI) from a Th(IV) matrix by branched butyl phosphates: insights from solvent extraction, chromatography and quantum chemical calculations. Sep Purif Technol 210:182–194. https://doi.org/10.1016/j.seppur.2018.08.005
Yan Z-Y, Huang Q-G, Wang L, Zhang F (2019) Synthesis of tailored bis-succinamides and comparison of their extractability for U(VI), Th(IV) and Eu(III). Sep Purif Technol 213:322–327. https://doi.org/10.1016/j.seppur.2018.12.039
Veliscek-Carolan J (2016) Separation of actinides from spent nuclear fuel: a review. J Hazard Mater 318:266–281. https://doi.org/10.1016/j.jhazmat.2016.07.027
Ansari SA, Mohapatra PK (2017) A review on solid phase extraction of actinides and lanthanides with amide based extractants. J Chromatogr A 1499:1–20. https://doi.org/10.1016/j.chroma.2017.03.035
Paiva AP, Malik P (2004) Recent advances on the chemistry of solvent extraction applied to the reprocessing of spent nuclear fuels and radioactive wastes. J Radioanal Nucl Chem 261:485–496. https://doi.org/10.1023/B:JRNC.0000034890.23325.b5
Chen LX, Wang Y, Yuan XY, Ren Y, Liu N, Yuan LH, Feng W (2019) Highly selective extraction of uranium from nitric acid medium with phosphine oxide functionalized pillar 5 arenes in room temperature ionic liquid (vol 192, pg 152, 2018). Sep Purif Technol 209:1027–1027. https://doi.org/10.1016/j.seppur.2018.10.017
Prathibha T, Venkatesan KA, Antony MP (2018) Comparison in the aggregation behaviour of amide extractant systems by dynamic light scattering and ATR-FTIR spectroscopy. Colloids Surf A 538:651–660. https://doi.org/10.1016/j.colsurfa.2017.11.035
Koubský T, Fojtíková J, Kalvoda L (2017) Radical degradation stability of ether linkage in N,N,N′,N’-tetraoctyldiglycolamide and related organic extractants: a density functional study. Prog Nucl Energy 94:208–215. https://doi.org/10.1016/j.pnucene.2016.07.010
Pahan S, Boda A, Ali SM (2015) Density functional theoretical analysis of structure, bonding, interaction and thermodynamic selectivity of hexavalent uranium (UO22+) and tetravalent plutonium (Pu4+) ion complexes of tetramethyl diglycolamide (TMDGA). Theor Chem Acc 134:16. https://doi.org/10.1007/s00214-015-1641-7
Zhang H, Ao Y-Y, Wang Y, Zhao S-J, Sun J-Y, Zhai M-L, Li J-Q, Peng J, Li H-B (2023) Effect of radiolysis of TODGA on the extraction of TODGA/n-dodecane toward Eu(III): an experimental and DFT study. Nucl Sci Tech 34:10. https://doi.org/10.1007/s41365-023-01198-z
Whittaker D, Geist A, Modolo G, Taylor R, Sarsfield M, Wilden A (2018) Applications of diglycolamide based solvent extraction processes in spent nuclear fuel reprocessing, part 1: TODGA. Solvent Extr Ion Exch 36:223–256. https://doi.org/10.1080/07366299.2018.1464269
Ansari SA, Wadawale AP, Verboom W, Mohapatra PK (2022) Isolation of single crystals of a homoleptic UO22+-diglycolamide complex from a room temperature ionic liquid: X-ray crystallography and complexation studies. New J Chem 46:950–954. https://doi.org/10.1039/d1nj05760j
Woodhead D, McLachlan F, Taylor R, Müllich U, Geist A, Wilden A, Modolo G (2019) Nitric acid extraction into a TODGA Solvent modified with 1-octanol. Solvent Extr Ion Exch 37:173–190. https://doi.org/10.1080/07366299.2019.1625201
Weßling P, Müllich U, Guerinoni E, Geist A, Panak PJ (2020) Solvent extraction of An(III) and Ln(III) using TODGA in aromatic diluents to suppress third phase formation. Hydrometallurgy 192:105248. https://doi.org/10.1016/j.hydromet.2020.105248
Mahanty BN, Raut DR, Mohapatra PK, Das DK, Behere PG, Afzal M (2014) Comparative evaluation of actinide ion uptake by polymer inclusion membranes containing TODGA as the carrier extractant. J Hazard Mater 275:146–153. https://doi.org/10.1016/j.jhazmat.2014.04.059
Panja S, Mohapatra PK, Tripathi SC, Gandhi PM, Janardan P (2012) A highly efficient solvent system containing TODGA in room temperature ionic liquids for actinide extraction. Sep Purif Technol 96:289–295. https://doi.org/10.1016/j.seppur.2012.06.015
Ansari SA, Pathak P, Mohapatra PK, Manchanda VK (2011) Aqueous partitioning of minor actinides by different processes. Sep Purif Rev 40:43–76. https://doi.org/10.1080/15422119.2010.545466
Zhu Z-X, Sasaki Y, Suzuki H, Suzuki S, Kimura T (2004) Cumulative study on solvent extraction of elements by N,N,N′,N′-tetraoctyl-3-oxapentanediamide (TODGA) from nitric acid into n-dodecane. Anal Chim Acta 527:163–168. https://doi.org/10.1016/j.aca.2004.09.023
Usuda S, Yamanishi K, Mimura H, Sasaki Y, Kirishima A, Sato N, Niibori Y (2014) Separation of Am and Cm by using TODGA and DOODA(C8) adsorbents with hydrophilic ligand-nitric acid solution. J Radioanal Nucl Chem 303:1351–1355. https://doi.org/10.1007/s10967-014-3481-7
Sasaki Y, Morita Y, Kitatsuji Y, Kimura T (2010) Mutual separation of actinides from middle lanthanides by the combination of two neutral donors, N,N,N′,N′-tetraoctyl-3,6-dioxaoctanediamide and N,N,N′,N′-tetraethyldiglycolamide. Chem Lett 39:898–899. https://doi.org/10.1246/cl.2010.898
Sasaki Y, Tsubata Y, Kitatsuji Y, Sugo Y, Shirasu N, Morita Y, Kimura T (2013) Extraction behavior of metal ions by TODGA, DOODA, MIDOA, and NTAamide extractants from HNO3 ton-dodecane. Solvent Extr Ion Exch 31:401–415. https://doi.org/10.1080/07366299.2013.800431
Sasaki Y, Morita Y, Kitatsuji Y, Kimura T (2010) Extraction behavior of actinides and metal ions by the promising extractant, N,N,N′,N′-tetraoctyl-3,6-dioxaoctanediamide (Dooda). Solvent Extr Ion Exch 28:335–349. https://doi.org/10.1080/07366291003680723
Sasaki Y, Choppin GR (1996) Solvent extraction of Eu, Th, U, Np and Am with N,N’-dimethyl-N, N’-dihexyl-3-oxapentanediamide and its analogous compounds. Anal Sci 12:225–230. https://doi.org/10.2116/analsci.12.225
Huang M, Yan Y, Feng W, Weng S, Zheng Z, Fu X, Liu P (2014) Controllable tuning various ratios of ZnO polar facets by crystal seed-assisted growth and their photocatalytic activity. Cryst Growth Des 14:2179–2186. https://doi.org/10.1021/cg401676r
Mayer I (1985) Charge, bond order and valence in the ab initio SCF theory. Chem Phys Lett 117(4):396
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33:580–592. https://doi.org/10.1002/jcc.22885
Xu D, Shah Z, Cui Y, Jin L, Peng X, Zhang H, Sun G (2018) Recovery of rare earths from nitric acid leach solutions of phosphate ores using solvent extraction with a new amide extractant (TODGA). Hydrometallurgy 180:132–138. https://doi.org/10.1016/j.hydromet.2018.07.005
Sun G-X, Han J-T, Bao B-R, Sun S-X (1998) Structural effect of N,N-dialkylamide in toluene on the extraction of uranium(VI). J Radioanal Nucl Chem 232:245–247. https://doi.org/10.1007/BF02383748
Peroutka AA, Galley SS, Shafer JC (2023) Elucidating the speciation of extracted lanthanides by diglycolamides. Coord Chem Rev 482:215071. https://doi.org/10.1016/j.ccr.2023.215071
Acknowledgements
This work was supported by the Natural Science Foundation of Shandong Province (ZR2022QB067).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, S., Peng, X., Luo, Y. et al. UO22+ extraction and mechanism by diglycolamide extractants with different ether-oxygen chain skeletons. J Radioanal Nucl Chem 333, 2421–2431 (2024). https://doi.org/10.1007/s10967-024-09472-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-024-09472-z