Abstract
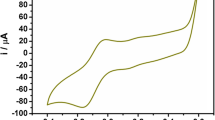
Novel iron(II) Schiff base type complexes of [Fe(4-benzyl-2-hydroxy-propiophenone)2(o-phenylenediamine)], [Fe(ninhydrin)2(ethylenediamine)], [Fe(ninhydrin)2(1,2-propylenediamine)], [Fe(2,4,6-trihydroxy-benzyl-4-methoxyphenyl-ketone)2(ethylenediamine)] and [Fe(ninhydrin)2(1,3-propylenediamine)] have been prepared by reaction of the components dissolved in ethanol at refluxing temperature in inert atmosphere, and were characterized by mass spectrometry (MS), infrared spectrometry (IR), thermal analysis, atomic force microscopy (AFM), 57Fe Mössbauer spectroscopy, cyclic voltammetry and biological activity tests. Mössbauer spectroscopy revealed the dominant occurrence of high spin FeII besides minor or no low spin FeII in the complexes, and minor FeIII impurity phase. The cyclic voltammograms indicated peaks due to redox processes in certain complexes. In these cases the occurrence of low spin FeII was also observed. Low biological activity was experienced for some compounds. The thermal decomposition process of all complexes was performed by TG–DTG–DTA analysis in air atmosphere aiming to understand the mechanism of the mass change with increasing temperature. The importance of the current research is to find correlation between the Fe spin states, redox processes and biological activity.








Similar content being viewed by others
References
Al-Shaalan NH (2011) Synthesis, characterization and biological activities of Cu(II), Co(II), Mn(II), Fe(II), and UO2(VI) complexes with a new Schiff base hydrazone: O-hydroxyacetophenone-7-chloro-4-quinoline hydrazone. Molecules 16:8629–8645
Qin W, Long S, Panunzio M, Biondi S (2013) Schiff bases: a short survey on an evergreen chemistry tool. Molecules 18:12264–12289
Gupta KC, Sutar AK (2008) Catalytic activities of Schiff base transition metal complexes. Coord Chem Rev 252:1420–1450
Liu X, Manzur C, Novoa N, Celedón S, Carrillo D, Hamon J-R (2018) Multidentate unsymmetrically-substituted Schiff bases and their metal complexes: Synthesis, functional materials properties, and applications to catalysis. Coord Chem Rev 357:144–172
Fátima Â, Pereira CP, Olímpio CRSDG, Oliveira BGF, Franco LL, Silva PHC (2018) Schiff bases and their metal complexes as urease inhibitors—a brief review. J Adv Res 13:113–126
Fabbrizzi L (2020) Beauty in chemistry: making artistic molecules with Schiff bases. J Org Chem 85:12212–12226
Reddy V, Patil N, Reddy T, Angadi SD (2008) Synthesis, characterization and biological activities of Cu(II), Co(II), Ni(II), Mn(II) and Fe(III) complexes with Schiff base derived from 3-(4-chlorophenoxymethyl)-4-amino-5-mercapto-1,2,4-triazole. E-J Chem 5(3):529–538
Kumar J, Rai A, Raj V (2017) A comprehensive review on the pharmacological activity of Schiff base containing derivatives. Org Med Chem Int J 1(3):1–14
Mahapatra K, Ghosh AK, De S, Ghosh N, Sadhukhan P, Chatterjee S, Ghosh R, Sil PC, Roy S (2020) Assessment of cytotoxic and genotoxic potentials of a mononuclear Fe(II) Schiff base complex with photocatalytic activity in Trigonella. BBA Gen Subj 1864:129503–129518
Lochenie C, Heinz J, Milius W, Weber B (2013) Iron(II) spin crossover complexes with diaminonaphthalene-based Schiff base-like ligands: mononuclear complexes. Dalton Trans 44:18065–18077
Kuzmann E, Homonnay Z, Nagy S, Nomura K (2011) Mössbauer spectroscopy. In: Vértes A, Nagy S, Klencsár Z, Lovas R, Rösch F (eds) Handbook of nuclear chemistry. Springer, New York
Gütlich P, Eckhard B, Trautwein AX (2011) Mössbauer spectroscopy and transition metal chemistry. Springer, New York
Vértes A, Burger K, Korecz L (1979) Mössbauer spectroscopy. Elsevier, Amsterdam
Greenwood NN, Gibb TC (1971) Mössbauer spectroscopy. Chapman & Hall, London
Gütlich P, Goodwin HA (eds) (2004) Spin crossover in transition metal compounds, I–III. Springer, Berlin
Gütlich P (1981) Spin crossover in iron(II)-complexes. In: Metal Complexes. Structure and Bonding (Conference Proceedings), vol 44. Springer-Verlag, Berlin, Heidelberg
Várhelyi Jr Cs, Kovács A, Nemcsok D, Németh Z, Kuzmann E, Vértes A, Vékey K, Várhelyi Cs, Pokol Gy (2007) Spectroscopic and thermal studies of [Fe(dioximato)2(amine)2] mixed chelates. J Coord Chem 60:379–392
Németh Z, Kuzmann E, Vértes A, Kovács A, Várhelyi Jr Cs, Várhelyi Cs (2008) Mössbauer study of [Fe(Dioximato)nL2] mixed coordination compounds. Hyperfine Interact 185:159–165
Kuzmann E, Lengyel A, Homonnay Z, Várhelyi Jr Cs, Klencsár Z, Kubuki S, Szalay R (2014) Mössbauer study of novel iron(II)-dioxime complexes with branched alkyl chains. Hyperfine Interact 226:181–185
Várhelyi Jr Cs, Kuzmann E, Homonnay Z, Lengyel A, Pokol Gy, Izvekov V, Szalay R, Kun A, Tomoia-Cotisel M, Covaci E, Garg VK, Olivera AC, Goga F (2015) Preparation and characterization of novel [Fe(methylisopropylglyoximato)2(amine)2] mixed chelates. J Radioanal Nucl Chem 304:745–750
Várhelyi Jr Cs, Lengyel A, Homonnay Z, Szalay R, Pokol Gy, Szilágyi IM, Huszthy P, Papp J, Goga F, Golban LM, Kuzmann E (2017) Mössbauer study of novel iron(II) complexes synthesized with Schiff bases. Hyperfine Interact 238:87
Várhelyi Jr Cs, Kuzmann E, Homonnay Z, Pokol Gy, Szilágyi IM, Huszthy P, Szalay R, Papp J, Goga F, Golban LM (2017) Synthesis of Fe(II)-complexes with Schiff bases, physical–chemical and biological activity study. Acta Scientiarum Transylvanica 25:87–94
Scholz F, Schroder U, Gulaboski R (2005) Electrochemistry of immobilized particles and droplets. Springer, Berlin
Aburas N, Lolić A, Stevanović N, Tripković T, Nikolić-Mandić S, Baoŝić R (2012) Electrochemical behavior and antioxidant activity of tetradentate Schiff bases and their copper(II) complexes. J Iran Chem Soc 9:859–864
Zappe L, Schönfeld S, Hörner G, Zenere KA, Leong CF, Kepert CJ, D’Alessandro DM, Weber B, Neville SM (2020) Spin crossover modulation in a coordination polymer with the redox-active bis-pyridyltetrathiafulvalene (py2TTF) ligand. Chem Commun 56:10469–10472
Naghiu MA, Gorea M, Mutch E, Kristály F, Tomoaia-Cotișel M (2013) Forsterite nanopowder: structural characterisation and biocompatibility evaluation. J Mater Sci Technol 29(7):628–632
Horovitz O, Tomoaia Gh, Mocanu A, Yupsanis T, Tomoaia-Cotișel M (2007) Protein binding to gold auto-assembled films. Gold Bull 40(4):295–304
Zdrenghea UV, Tomoaia Gh, Pop-Toader DV, Mocanu A, Horovitz O, Tomoaia-Cotișel M (2011) Procaine effect on human erythrocyte membrane explored by atomic force microscopy. Comb Chem High Throughput Screen 14(4):237–247
CsP R, Rácz LZ, Floare CG, Tomoaia Gh, Horovitz O, Riga S, Kacso I, Borodi Gh, Sárközi M, Mocanu A, Roman C, Tomoaia-Cotișel M (2023) Curcumin and whey protein concentrate binding: thermodynamic and structural approach. Food Hydrocolloids 139:1085–1147
Khezri A, Edjlali L, Es’ haghi M, Vardini MT, Basharnavaz H (2023) Addition of Schiff bases to hybrid silane sol–gel coatings: an efficient strategy to develop an active system for corrosion protection of copper. J Mater Eng Perform 1–10
Smirnova E, Ankudinov A, Chepurnaya I, Timonov A, Karushev M (2023) In-situ EC-AFM study of electrochemical p-doping of polymeric nickel(II) complexes with Schiff base ligands. Inorganics 11:41
Sela A, Cohen E, Avram L, Rodov V, Poverenov E (2023) Solvent-free synthesis of polysaccharide derivatives via heterogeneous Schiff base chemistry. Green Chem 25:922
Chiericato G Jr, Saldanha-Silva AP, Guinesi LS (2004) Synthesis, characterization and electropolymerization of a new polypyrrole iron(II) Schiff-base complex. Polyhedron 23:1953–1960
Qian YLC, Li Y, Yang Y, Lin D, Liu X, Chen C (2021) Syntheses, crystal structures of two Fe(III) Schiff base complexes with chelating o-vanillin aroylhydrazone and exploration of their bio-relevant activities. J Inorg Biochem 218:111405
Klencsár Z, Kuzmann E, Vértes A (1996) User-friendly software for Mössbauer spectrum analysis. J Radioanal Nucl Chem 210:105–118
Beveridge TJ (2001) Use of the gram stain in microbiology. Biotech Histochem 76(3):111–118
Stewart EJ, Madden R, Paul G, Taddei F (2005) Aging and death in an organism that reproduces by morphologically symmetric division. PLoS Biol 3(2):e45
Bartyzel A (2017) Synthesis, thermal study and some properties of N2O4—donor Schiff base and its Mn(III), Co(II), Ni(II), Cu(II) and Zn(II) complexes. J Therm Anal Calorim 127:2133–2147
Nakamoto K (1997) Infrared and Raman spectra of inorganic and coordination compounds, part B, 5th edn. Wiley, New York
Stevens JG, Stevens VE (1966–1979) Mössbauer Effect Data Index (MERDI). Adam Hilger, London
Bard AJ, Faulkner LR (1980) Electrochemical methods: Fundamentals and applications. Wiley, New York
Schönfeld S, Dankhoff K, Baabe D, Zaretzke MK, Bröring Schötz MK, Köhler A, Hörner G, Weber B (2020) Iron(II) spin crossover complexes based on a redox active equatorial Schiff-base-like ligand. Inorg Chem 59(12):8320–8333
Novak I, Komorsky-Lovrić Š, Žunec S, Vrdoljak AL (2013) Anodic behaviour of some bispyridinium oximes on a glassy carbon electrode. Int J Electrochem Sci 8:8918–9834
Chum HL, Dockal ER, Rabockai T (1975) Electrochemistry of organometallic compounds I. Cyclic voltammetry of organocobaloximes in aqueous acid solutions. J Electroanal Chem 63:197–205
Ferreira H, Conradie MM, Conradie J (2019) Electrochemical and electronic properties of a series of substituted polypyridine ligands and their Co(II) complexes. Inorg Chim Acta 486:26–35
Ferreira H, Conradie MM, Conradie J (2019) Cyclic voltammetry data of polypyridine ligands and Co(II)-polypyridine complexes. Data Brief 22:436–445
Reddy KH, Babu MS, Babu PS, Dayananda S (2004) Synthesis, characterization and nuclease activity of copper(II), nickel(II), Co(II) and iron(II) complexes with oxime-thiosemicarbazones. Indian J Chem 43A:1233–1238
Niufar NN, Haycock FL, Wesemann JL, MacStay JA, Heasley VL, Kovacic P (2002) Reduction potentials of conjugated aliphatic ketones, oximes, and imines: correlation with structure and bioactivity. Revista de la Sociedad Química de México 46(4):307–312
Frei A, Zuegg J, Elliott AG, Baker M, Braese S, Brown C, Chen F, Dowson CG, Dujardin G, Jung N, King AP, Mansour AM, Massi M, Moat J, Mohamed HA, Renfrew AK, Rutledge PJ, Sadler PJ, Todd MH, Willans CE, Wilson JJ, Cooper MA, Blaskovich MAT (2020) Metal complexes as a promising source for new antibiotics. Chem Sci 11:26272639
Acknowledgements
The authors wish to express their thankfulness to the “Domus Hungarica Foundation” of Hungary for the several fellowships provided to Csaba Várhelyi, Jr. We are grateful for the support by grants from the Hungarian National Research, Development and Innovation Office for the OTKA projects.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Várhelyi, C., Szőke, Á., Sziráki, L. et al. Fe spin states and redox processes in Schiff base type complexes. J Radioanal Nucl Chem 332, 4125–4139 (2023). https://doi.org/10.1007/s10967-023-09095-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-09095-w