Abstract
The aim of our work was to develop a generally applicable liquid chromatographic method for the determination of the residue free 68Ga3+ content of labeling reaction mixtures, in order to facilitate comparative labeling experiments in the early stages of chelator development. We achieved the retention of free 68Ga3+ on a mixed mode cation exchanger HPLC column with a gradient program, which allowed the separation from the non-retained complexes. The effects of reaction conditions (time, temperature) and the application of non-coordinating buffers were examined. Furthermore, recovery of free 68Ga3+ and these complexes were investigated, values of which were 94.9–97.4% and 87.4–99.6%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
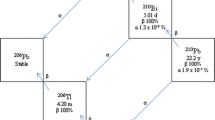
68Ga became the most frequently used diagnostic radiometal in Positron Emission Tomography (PET) imaging- and preclinical research by now [1,2,3]. This is probably not related to its rather short half-life (67.7 min) and non-ideal imaging properties (1899 keV max. positron energy for 68Ga—vs. 633.5 keV [4, 5] for 18F causing 3 × higher mean positron range in soft tissue, compared to 18F). It is most likely caused by its convenient availability from 68Ge/68Ga generators, which boosted the application of gallium labeled somatostatin- and Prostate-Specific Membrane Antigen (PSMA) tracers in the clinic, as well as the development of countless gallium tracers in preclinical research. The constantly increasing demand facilitated the development of cyclotron production methods by the proton irradiation of enriched 68Zn targets in solution- and in solid form [6, 7]. Much work was done in the field of chelator development for 68Ga in the last decade [8, 9]. Several chelators with high selectivity and stability were developed, but the new tracers are often tested first with the „good enough” 2,2′,2″,2″′-(1,4,7,10-tetraazacyclododecane-1,4,7,10-tetrayl)tetraacetic acid (DOTA, see Fig. 12. in SI) and continue their career with this less-than-ideal chelator. Replacement of the chelator after the first promising biodistribution- or imaging results just is not worth the effort, although using a chelate with different polarity has the potential to improve the in-vivo properties of the tracer [10]. Altering the polarity and charge of the complex by changing the chelator can modify biodistribution and the route- and rate of excretion. Besides intellectual property related issues, two technical difficulties are hampering the use of non-DOTA chelators in tracer development. The first is the conjugation of the bifunctional chelator to the targeting vector. This can be easily overcome by the commercial availability of protected bifunctional chelators. The second problem is the lack of experience in labeling these chelators, which should be practiced first, before using them as an in-vivo tool. A logical idea is to perform labeling of the free chelator but following the labeling reaction is usually problematic. Development of a TLC or HPLC method for non-conjugated chelator labeling can be time consuming, as the separation of a highly polar metal complex and the free metal is often challenging.
Despite the sporadic clinical use of more advanced chelators, inorganic chemists continue the work to develop new ligands for radiometal complexation. This is a rather lengthy process, often involving several trial-and-error cycles. The kinetic- and equilibrium methods applied in the classic chemical concentration range can have some predictive value during this process, but the decisive experiments must be done with radioisotopes. The synthesis of new chelators is often challenging, and their modification to bifunctional chelators is even more labor intensive, thus it is important to be able to check their applicability in the early stage of development with comparative radiolabeling experiments. Usually, the RCY of the labeling reaction is compared with that of a reference chelator (e.g. DOTA) among constant labeling conditions (eg.: pH 4 for gallium, 5 min., 95 °C). Performing the labeling at various chelator concentrations allows the quick determination of the minimum concentration, necessary for quantitative labeling. This provides information about the selectivity of a new chelator, compared to the reference. If the charge- and polarity of the radiometal complex is different from the reference, a new analytical method might be necessary. At present, reversed phase HPLC methods are generally used to follow the labeling reactions, utilizing the retention of the chelator-conjugated biomolecule to separate it from free metal ions [11, 12]. These methods are not suitable for following the labeling of highly polar free chelators with low retention on apolar columns. The retention of the residue free radiometal would simplify the conduction of comparative radiolabeling work, thus we aimed to develop a HPLC separation method for free 68Ga3+ and various unconjugated gallium complexes.
Mixed-mode chromatographic stationary phases allow the use of more than one interaction (reverse phase and ion-exchange separation) to separate sample components [12, 13]. In addition to apolar chains, they also contain cation- and/or anion-exchange groups, so multiple interactions can be used during one separation. Appearance of new generation of mixed-mode stationary phases and the better understanding of multiple interactions drives their broader application. These are excellent for separating polar- and charged molecules [14], having low retention on reversed phase. The core–shell technology [15,16,17] gives the opportunity to achieve high kinetic efficiency at a higher flow rate, while generating the pressure available with conventional HPLC systems, the separation speed can be increased.
Radiolabeling reactions are often followed by thin-layer chromatography, however, Larenkov et al. showed that HPLC results do not always match those obtained by TLC [18, 19]. An interesting effect was observed during the development of methods for the investigation of the radiochemical composition of various 68Ga-containing radiopharmaceuticals by glass microfiber chromatography paper impregnated with silica gel thin-layer chromatography (iTLC), which is a porous, slightly acidic paper that provides excellent resolution. In some cases HPLC significantly underestimated the free 68Ga3+ content of the samples, compared to TLC. The comparison of test results, obtained with different TLC and HPLC methods showed, that a part of the ionic 68Ga was irreversibly adsorbed on reversed-phase columns up to 10%. Without the use of a column, the entire amount of injected activity was recovered with an error of 1.5% in the tested samples, confirming, that the adsorption occurs mainly on the column. Hacht's measurements [20] indicate that [68Ga]Ga-acetate associates can be formed at low gallium ion concentrations and relatively high acetate concentrations (0.07–0.2 M), which can be retained on the column. It was assumed, that the retention of such associates can be reduced if acetonitrile is added to the eluent, however, the opposite result was obtained, namely the activity loss measured on the chromatographic column increased when acetonitrile was added to the HPLC eluent. 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES) [21] is another frequently used buffer for radiolabeling. In contrast to acetate buffered solutions, samples containing non-coordinating HEPES (pH 3–7) was characterized by the gradual formation of colloidal 68Ga, until the complete hydrolysis of all ionic 68Ga. The few sources found in the literature [18, 19, 22, 23] show that the 68Ga3+ cations can stick to a significant extent on a reversed-phase HPLC column, so it does not provide the necessary reliability for the analysis of radiopharmaceuticals. This is especially important when dealing with radiopharmaceuticals that are not subjected to further purification (e.g. by solid phase extraction).
Experimental
Chemicals and reagents
All reagents and solvents were obtained from Sigma-Aldrich and VWR. Enriched zinc-68 (98.60%) was obtained from NeonestAB (Stockholm, Sweden). Ultrapure hydrochloric acid (35%) was supplied by Carl-Roth GmbH, the HCl solutions were prepared from this by dilution with MilliQ Type-1 water. 2,2′,2″,2″′-(1,4,7,10-tetraazacyclododecane-1,4,7,10-tetrayl)tetraacetic acid (DOTA) and 2,2′,2″-(1,4,7-triazacyclononane-1,4,7-triyl)triacetic acid (NOTA) was purchased from ChemaTech (Dijon, France), 6-amino-6-methylperhydro-1,4-diazepine tetra acetic acid (AAZTA, see Fig. S12. in SI) and its derivative (AAZTA-C4, see Fig. S12. in SI) were provided to us by Zsolt Baranyai. TK400 resin was purchased from TrisKem (Bruz, France). HPLC grade methanol (Fisher Solutions) and MilliQ water were used as eluent. Glass microfiber chromatography paper impregnated with silica gel (iTLC-SG) was supplied by Agilent Technologies.
Cyclotron production of 68Ga radionuclide
68Ga isotope was produced in a GE PETtrace cyclotron with proton irradiation of solid zinc target (68Zn(p,n)68Ga). The target was prepared by pressing approximately 40 mg enriched zinc-68 powder to form a pellet and this pellet was pressed into an aluminum target holder. Targets were irradiated with 50 µA proton beam for 10 min. Purification: irradiated target (approx. 5 GBq activity) was dissolved in 10 mL 5 M u.p. HCl and loaded to Zr resin, washed with 10 mL 5 M HCl and eluted to TK200 resin with 5 mL 2 M HCl. Final elution from the second resin was done with 0.05 M HCl.
Equipment and conditions
68Ga radioisotope was obtained from a 68Ge/68Ga isotope generator (Eckert-Ziegler, GalliaPharm®, Berlin, Germany, eluent: 0.1 M ultra-pure HCl) for the HPLC method development. Gamma spectrometry was performed on a Canberra HPGe detector. Activities were measured with Capintec CRC-15PET dose calibrator and Perkin Elmer Packard Cobra gamma counter (Llantrisant, UK). For pH adjustment Mettler Toledo MP220 pH meter with an InLab 413/IP67 combined pH electrode was used. Thin layer chromatographic measurements were evaluated on a Raytest miniGita Star Radio-TLC scanner (Beta Detector GMC) by GINA Star TLC software using 0.05 M Na2CO3 as the mobile phase.
Chromatographic experiments were performed on a Waters Acquity UPLC I-Class System equipped with Binary Solvent Manager, a Sample Manager (Flow-Through-Needle Injector with 100 µL loop), a Column Manager, a Photodiode Array Detector. PDA detector was part of the system, however, due to the very small amount of substances involved in the reactions, we did not use it during the measurements. A photomultiplier tube (Hamamatsu Photonics), equipped with a plastic scintillator was used as radioactivity detector. Data were evaluated by Empower 3 chromatography software. The separation was achieved with a Coresep 100 (Sielc Technologies) RP-SCX 4.6 × 50 mm, 90 Å, 2.7 µm column, with a gradient program (To the eluent, ammonium acetate is dissolved in water and acidified to pH 4, then oxalic acid of pH 3 is added; 0.5 M pH 4 AmAc, 0.005 M pH 3 oxalic acid–water, 0:100 (v/v) for 1.5 min and then increased to 100:0 (v/v) for 2 min, followed by an isocratic regime for 2 min). The chromatographic method lasted for 5 min. Experiments were carried out at a flow rate of 0.6 mL/min and volume of injection depended on the radioactive concentration in the range of 5–10 µL. Higher flowrates were not tested in order to keep the method transferable to HPLC systems with lower pressure limit. For experiments on a microfluidic system (Fig. S8. in Supplementary Information), the same method was used at a flow rate of 0.4 mL/min. The flow rate was decreased to keep the pressure under the limit of the plastic injector valve and fittings.
Sample preparation procedure
Labeling in Eppendorf tubes: Chelators were labeled in 1.5 mL Eppendorf tubes at 95 °C in constant temperature heating block with reaction times of 5 min. Samples were prepared by mixing 80 μL of pH 4.0 buffer solution, 10 μL of chelator solution (0.1–100 μM), and 10 μL of acidic solution of 68Ga radiometal. The radioactivity of the sample was ~ 0.15–0.40 MBq per reaction.
Microfluidic sample: Samples were prepared by mixing 25 μL of ammonium acetate buffer (2 M; pH 4.0) and 25 μL of chelating solution (0.1–100 μM) which was placed in the appropriate port on the microfluidic system. 50 μL of acidic solution of 68Ga radiometal was placed in 2. syringe. Reactions were performed at room temperature or 100 °C for 15 s to 5 min in a PEEK tube.
Results and discussion
Radio-HPLC method development
Our aim was to achieve the retention of free metal isotope and separation from the metal-chelate. We used Coresep 100 column (Sielc Technologies, RP-SCX 4.6 × 50 mm, 90 Å, 2.7 µm) which is a reversed-phase cation-exchange column with core–shell particles, this is shown in the Fig. 1. It has C12 carbon chain and carboxylic acid functional group with a pKa of 2.
[68Ga]Ga3+ and [68Ga]Ga-DOTA were used during the method development [24] (Fig. 2). We chose the following chromatographic conditions to achieve retention of free, uncomplexed [68Ga]Ga3+. The column was equilibrated with pure water to enable retention of free [68Ga]Ga3+ and elution of [68Ga]Ga-DOTA, then quickly switched with a gradient program (0.5 M; pH 4 AmAc, 0.005 M pH 3 oxalic acid–water, 0:100 (v/v) for 1.5 min and then increased to 100:0 (v/v) for 2 min, followed by an isocratic regime for 2 min) to 0.5 M ammonium-acetate buffer (pH 4), containing 5 mM oxalic acid to elute the adsorbed free [68Ga]Ga3+.
Among these conditions the neutral [68Ga]Ga-NOTA complex had higher retention, than the negatively charged [68Ga]Ga-DOTA, so the two complexes could be baseline separated with 1.7 resolution (Fig. 3).
The method was also tested with the following chelators: AAZTA, AAZTA-C4, NO2BzDOTA, HXTA, NOPO, TRAP, DTPA and EDTA (chromatograms in SI, Fig. S13–20.).
HPLC separation of radiometal containing samples is usually problematic, as the adsorption of carrier free metal cations on solid surfaces of the HPLC system components and the column can alter the results. The use of excess open-chain chelator in the eluent can decrease this adsorption effect. We used oxalic acid to remove adsorbed [68Ga]Ga3+ from the column. In order to determine the recovery of the gallium activity eluted from the column (Table 1), we collected the effluent and measured its activity with gamma counter. We found approx. 8, 5 and 3% loss in the case of 10, 5 and 1 µL injected volume, respectively. Radioactivity detector linearity was determined with repeated injections of [68Ga]Ga-DOTA. Due to the decay of the 68Ga isotope, repeated injection of the same sample results in gradually lower peak areas. The activity of the injected sample was calculated to the time of detection. Peak area was plotted against the activity to create a calibration curve. Calibration was not repeated for all radioactive species. Composition of a radioactive samples were determined as peak area%, as the same radioisotope was detected in each component, so the same calibration curve was considered valid for all compounds.
The method also met the following validation criteria (Table 2). Linearity of the radioactivity detector was 0.9914 measured by the mentioned chromatographic method above with [Ga68]Ga-DOTA (Fig. S9. in Supplementary Information).
We compared the results of microfluidic and manual labeling experiments, and we found similar results (Fig. 4).
DOTA labeling with [68Ga]Ga3+ in Eppendorf tubes and capillary reactor (Microfluid system: 25 μL of AmAc buffer (2 M; pH 4.0) and 25 μL of DOTA, 50 μL of [68Ga]Ga3+ in 0.05 M HCl, 100 °C, 5 min. Manual labeling: 80 μL of AmAc buffer (1 M; pH 4.0), 10 μL DOTA, 10 μL of [68Ga]Ga3+ in 0.05 M HCl, 95 °C, 5 min.)
AAZTA and AAZTA-C4, belonging to the semi-macrocyclic compound family, also have excellent complex-forming properties at room temperature, so they were used for further testing of the microfluidic system [26, 27]. Figure 5 shows the concentration dependence of the formation of the two gallium complexes as a function of the amount of ligands during a reaction time of 5 min. In the case of AAZTA, the radiochemical yield is already over 22% at a concentration of 0.75 μM, while the degree of complex formation does not even reach 14% for the derivative with an alkyl chain. However, in the presence of chelating agents of 7.5 μM and more, the two curves overlap, and the radiochemical yields are almost the same.
The formation of the [68Ga]Ga-AAZTA-C4 complex seemed to be slower and slightly more inhibited based on the concentration dependence presented above, therefore we investigated the time dependence of the formed complexes at chelator concentrations of 25 μM, 7.5 μM and 2.5 μM (Fig. 6). In the presence of 25 and 7.5 μM AAZTA-C4 complexing agent, saturation can be observed already around 60 s and the radiochemical yield values did not increase significantly. However, at the smallest concentration value tested, the rate of complex formation slowed down dramatically, a continuous rise of the curve can be observed up to 180 s.
Finally, using the microfluidic system, we compared the rate of complex formation of the two investigated ligands at a chelator concentration of 7.5 μM. In the concentration dependence shown in Fig. 5, the two AAZTA derivatives resulted in the same radiochemical yield at a concentration value of 7.5 μM after a reaction time of 5 min, which is why we thought of performing a more detailed investigation of this measurement point. In Fig. 7, we obtained the expected result, that although the curves converge in the 5th minute, but until then, AAZTA is able to form a gallium complex faster than its derivative with an alkyl chain.
Conclusions
We developed a generally applicable liquid chromatographic method for the separation of non-conjugated chelators labeled with 68Ga. This method can facilitate the determination of optimal labeling conditions in the case of new chelators- and offer a straightforward way for the comparison of different chelators. The mixed-mode nature of the applied stationary phase implies the potential of further development of the separation to chelator-peptide conjugates, where the eluent pH, ionic strength and organic solvent content can be varied to achieve efficient separation.
The in-house developed microfluidic synthesis module used in combination with the newly developed separation method, can provide highly reproducible results to determine concentration- and temperature dependence of labeling reactions.
References
Hennrich U, Benešová M (2020) Pharmaceuticals 13:38
Kilian K (2014) Rep Pract Oncol Radiother 19:S13–S21
Notni J, Wester H-J (2018) J Label Compd Radiopharm 61:141–153
Sanchez-Crespo A (2013) Appl Radiat Isot 76:55–62
Coenen HH, Ermert J (2021) Nucl Med Biol 92:241–269
Nelson BJB, Wilson J, Richter S, Duke MJM, Wuest M, Wuest F (2020) Nucl Med Biol 80–81:24–31
Pandey MK, Byrne JF, Jiang H, Packard AB, DeGrado TR (2014) Am J Nucl Med Mol Imaging 4:303–310
Spang P, Herrmann C, Roesch F (2016) Semin Nucl Med 46:373–394
Roesch F, Riss PJ (2010) Curr Top Med Chem 10:1633–1668
Banerjee SR, Pomper MG (2013) Appl Radiat Isot 76:2–13
Mueller D, Breeman WAP, Klette I, Gottschaldt M, Odparlik A, Baehre M, Tworowska I, Schultz MK (2016) Nat Protoc 11:1057–1066
Kristl A, Podgornik A, Pompe M (2021) J Chromatogr B 1171:122557
Orlovsky V, Zelechonok Y (2011) Chromatogr Today 1:24–28
Forgács V, Fekete A, Gyuricza B, Szücs D, Trencsényi G, Szikra D (2022) Pharmaceuticals 15(2):147
González-Ruiz V, Olives AI, Martín MA (2015) TrAC Trends Anal Chem 64:17–28
Liu Y, Sun K, Shao C, Shi X, Zeng J, Guo R, Zhang B (2021) J Chromatogr A 1648:462218
Poole CF, Atapattu SN (2020) J Chromatogr A 1634:461692
Larenkov AA, Maruk AY, Kodina GE (2018) Radiochemistry 60:625–633
Maruk AY, Larenkov AA (2020) J Radioanal Nucl Chem 323:189–195
Hacht B (2016) Open Sci J 1(1):1–13
Martins AF, Prata MIM, Rodrigues SPJ, Geraldes CFGC, Riss PJ, Amor-Coarasa A, Burchardt C, Kroll C, Roesch F (2013) Contrast Media Mol Imaging 8:265–273
Nelson BJB, Andersson JD, Wuest F et al (2022) EJNMMI Radiopharm Chem 7:27
Gillings N, Todde S, Behe M et al (2020) EJNMMI Radiopharm Chem 5:7
Baranyai Z, Tircsó G, Rösch F (2020) Eur J Inorg Chem 2020:36–56
Manzoni L et al (2012) ChemMedChem 7:1084–1093
Sinnes JP, Nagel J, Rösch F (2019) EJNMMI Radiopharm chem 4:18
Kandegedara A, Rorabacher DB (1999) Anal Chem 71:3140–3144
Vágner A et al (2018) Front Chem 6:170
Funding
Open access funding provided by University of Debrecen.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Forgács, V., Jószai, I., Fekete, A. et al. HPLC method development using a mixed-mode column for following 68Ga-radiolabeling. J Radioanal Nucl Chem 332, 3571–3577 (2023). https://doi.org/10.1007/s10967-023-09031-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-09031-y