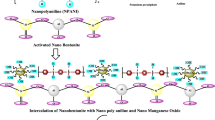
Abstract
Separation of thorium, uranium, and rare earth elements (REEs) requires selective materials with high capacity, good thermal, chemical and radiation resistance. For this aim, Zr, Si and Ti oxide nanoparticles (NPs) have been prepared using metal alkoxide precursors via hydrothermal method. Polyacrylonitrile (PAN) was used as a supporting material to obtain nanocomposite (NC) beads for the potential use in industrial scale applications. The surface characteristics and morphological properties of nano metal oxide powders and composites were determined. Metal uptake and separation studies of thorium and REEs were carried out from sulfuric acid leach solution of Beylikova ore.










Similar content being viewed by others
References
Altas Y, Tel H, Inan S, Sert S, Cetinkaya B, Sengul S, Ozkan B (2018) An experimental design approach for the separation of thorium from rare earth elements. Hydrometallurgy 178:97–105
Tel H, Altaş Y, Eral M, Sert Ş, Çetinkaya B, İnan S (2010) Preparation of ZrO2 and ZrO2–TiO2 microspheres by the sol–gel method and an experimental design approach to their strontium adsorption behaviours. Chem Eng J 161:151–160
İnan S, Tel H, Sert Ş, Çetinkaya B, Sengül S, Özkan B, Altaş Y (2018) Extraction and separation studies of rare earth elements using Cyanex 272 impregnated Amberlite XAD–7 resin. Hydrometallurgy 181:156–163
Sert Ş, Altaş Y, Tel H, İnan S, Çetinkaya B, Sengül S, Özkan B (2019) Investigation of sorption behaviors of La, Pr, Nd, Sm, Eu and Gd on D2EHPA–impregnated XAD7 resin in nitric acid medium. Sep Sci Technol 56:26–35
Aslani CK, Amik O (2021) Active Carbon/PAN composite adsorbent for uranium removal: Modeling adsorption isotherm data, thermodynamic and kinetic studies. Appl Radiat Isot 168:109474
Li M, Jin Z, Zhang W, Bai Y, QiangCao Y, Li W, Wu D, Li A (2019) Comparison of chemical stability and corrosion resistance of group IV metal oxide flms formed by thermal and plasma–enhanced atomic layer deposition. Nature 9:10438
Gao Y, Yuan Y, Ma D, Li L, Li Y, Xu W, Tao W (2014) Removal of aqueous uranyl ions by magnetic functionalized carboxymethylcellulose and adsorption property investigation. J Nucl Mater 453(1–3):82–90
Mahmoud ME, Saad EA, El-Khatib AM, Soliman MA, Allam EA (2018) Adsorptive removal of radioactive isotopes of cobalt and zinc from water and radioactive wastewater using TiO2/Ag2O nanoadsorbents. Prog Nucl Energy 106:51–63
Guo G-Y, Chen Y-L (2005) A nearly pure monoclinic nanocrystalline zirconia. J Solid State Chem 178(5):1675–1682
Mahdavi S, Amini N, Merrikhpour H, Akhzari D (2017) Characterization of bare and modified nano-zirconium oxide (ZrO2) and their applications as adsorbents for the removal of bivalent heavy metals. Korean J Chem Eng 34:234–244
Pacheco S, Tapia J, Medina M, Rodriguez R (2006) Cadmium ions adsorption in simulated wastewater using structured alumina–silica nanoparticles. J Noncryst Solids 352(52–54):5475–5481
Takami S, Sato T, Mousavand T, Ohara S, Umetsu M, Adschiri T (2007) Hydrothermal synthesis of surface-modified iron oxide nanoparticles. Mater Lett 61:4769–4772
Hu J, Lo I, Chen G (2007) Performance and mechanism of chromate (VI) adsorption by δ-FeOOH-coated maghemite (γ-Fe2O3) nanoparticles. Sep Purif Technol 58:76–82
İnan S, Altaş Y (2011) Preparation of zirconium–manganese oxide/polyacrylonitrile (Zr–Mn oxide/PAN) composite spheres and the investigation of Sr(II) sorption by experimental design. Chem Eng J 168:1263–1271
Wang N, Li J, Lv W, Feng J, Yan W (2015) Synthesis of polyaniline/TiO2 composite with excellent adsorption performance on acid red G. RSC Adv 5:21132–21141
Feng J, Chen J, Wang N, Li J, Shi J, Yan W (2016) Enhanced adsorption capacity of polypyrrole/TiO2 composite modified by carboxylic acid with hydroxyl group. RSC Adv 6:42572–42580
Leun D, SenGupta AK (2000) Preparation and characterization of magnetically active polymeric particles (MAPPs) for complex environmental separations. Environ Sci Technol 34(15):3276–3282
Shubha KP, Raji C, Anirudhan TS (2001) Immobilization of heavy metals from aqueous solutions using polyacrylamide grafted hydrous tin(IV) oxide gel having carboxylate functional groups. Water Res 35(1):300–310
Cumbal LH, SenGupta AK (2005) Preparation and characterization of magnetically active dual-zone sorbent. Ind Eng Chem Res 44(3):600–605
Ma W, Ya FQ, Han M, Wang R (2007) Characteristics of equilibrium, kinetics studies for adsorption of fluoride on magnetic-chitosan particle. J Hazard Mater 143(1–2):296–302
Nilchi A, Atashi H, Javid AH, Saberi R (2007) Preparations of PAN-based adsorbers for separation of cesium and cobalt from radioactive wastes. Appl Radiat Isot 65:482–487
Nilchi A, Saberi R, Moradi M, Azizpour H, Zarghami R (2011) Adsorption of cesium on copper hexacyanoferrate–PAN composite ion exchanger from aqueous solution. Chem Eng J 172:572–580
Cakir P, Inan S, Altas Y (2014) Investigation of strontium and uranium sorption onto zirconium-antimony oxide/polyacrylonitrile (Zr–Sb oxide/PAN) composite using experimental design. J Hazard Mater 271:108–119
Xu J, Virolainen S, Zhang W, Kuva J, Sainio T, Koivula R (2018) Polyacrylonitrile-encapsulated amorphous zirconium phosphate composite adsorbent for Co, Nd and Dy separations. Chem Eng J 351:832–840
Martin DM, Jalaff LD, Garcia MA, Faccini M (2019) Selective recovery of europium and yttrium ions with Cyanex 272-polyacrylonitrile nanofibers. Nanomaterials 9(12):1648
Rehan M, Lai X, Kale GM (2011) Hydrothermal synthesis of titanium dioxide nanoparticles studied employing in situ energy dispersive X–ray diffraction. CrystEngComm 13:3725–3732
Ahmad T, Shahazad M, Phul R (2017) Hydrothermal synthesis, characterization and dielectric properties of zirconia nanoparticles. Mater Sci Eng Int J 1(3):100–104
Niu B, Wang X, Wu K, He X, Zhang R (2018) Mesoporous titanium dioxide: synthesis and applications in photocatalysis. Materials 11:1910
Liu Z, Zhou Q, Hong Z, Xu R (2017) Effects of surface charge and functional groups on the adsorption and binding forms of Cu and Cd on roots of indica and japonica rice cultivars. Front Plant Sci 8:1489
Xu P, Wang H, Tong R, Du Q, Zhong W (2006) Preparation and morphology of SiO2/PMMA nanohybrids by microemulsion polymerization. Colloid Polym Sci 284:755–762
Keramati H, Saidi MH, Zabetian M (2015) Stabilization of suspension of zirconia microparticles using nanoparticle halos mechanism: zeta potential effect. J Dispers Sci Technol 37:6–13
Liao DL, Wu GS, Liao BQ (2009) Zeta potential of shape-controlled TiO2 nanoparticles with surfactants. Colloids Surf A Physicochem Eng Asp 348:270–275
Lu GW, Gao P (2010) Emulsions and microemulsions for topical and transdermal drug delivery. In: Kulkarni Vitthal S (ed) Handbook of non-invasive drug delivery systems. Elsevier, Oxford, pp 59–94
Joseph E, Singhvi G (2019) Multifunctional nanocrystals for cancer therapy: a potential nanocarrier. In: Alexandru MG (ed) Nanomaterials for drug delivery and therapy. Elsevier, Bucharest, pp 91–116
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Özkan, B., Altaş, Y. & İnan, S. Investigation of nanocomposite efficiency on the separation and purification processes of thorium and rare earth elements. J Radioanal Nucl Chem 332, 4677–4686 (2023). https://doi.org/10.1007/s10967-023-08855-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-08855-y