Abstract
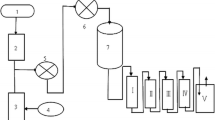
The paper describes the structure and iodine adsorption performance of Ag+ ion-exchange 13X molecular sieve (Ag-13X) with a small Si/Al ratio. The Ag-13X has a high specific surface area (408 m2/g) and a large amount of micropore volume (0.18 cm3/g). The Ag-13X presents a strong affinity with I2 and CH3I. The I2 static adsorption capacity of the 26.7% Ag zeolite reached 700 mg/g. The adsorption capacities under 1‰ and 1% CH3I feeds are up to 104.4 mg/g and 131 mg/g, respectively. This study indicates the applicability of Ag-13X in severe nuclear accidents.









Similar content being viewed by others
References
Nishimura T, Hoshi H, Hotta A (2015) Current research and development activities on fission products and hydrogen risk after the accident at Fukushima Daiichi nuclear power station. Nuclear Eng Technol 47:1–10
Gouello M (2012) Iodine Chemistry and aerosol composition in the reactor coolant system of a nuclear power plant in case of a severe accident; Chimie de l’iode et composition des aerosols dans le circuit primaire d’un reacteur nucleaire en situation d’accident grave
Wren D (2020) Radiation and Fission Product Transport in the RCS. Fission product transport processes in Reactor Accidents. CRC Press, pp 465–476
Jubin R, Strachan D, Soelberg N (2013) Iodine pathways and off-gas stream characteristics for aqueous reprocessing plants–a literature survey and assessment
Azambre B, Chebbi M (2017) Evaluation of silver zeolites sorbents toward their ability to promote stable CH3I storage as AgI precipitates. ACS Appl Mater Interfaces 9:25194–25203. https://doi.org/10.1021/acsami.7b02366
Haefner D (2007) Methods of gas phase capture of iodine from fuel reprocessing off-gas: a literature survey
Clément B, Cantrel L, Ducros G, Funke F, Herranz L, Rydl A, Weber G, Wren C (2007) State of the art report on iodine chemistry. Organisation for economic co-operation and development
International Atomic Energy A (2014) Treatment of radioactive gaseous waste. International Atomic Energy Agency (IAEA)
Jacquemain D OECD/NEA/CSNI status report on filtered containment venting. OECD, Report (2014) NEA/CSNI/R
Chien CC, Huang YP, Wang WC, Chao JH, Wei YY (2011) Efficiency of moso bamboo charcoal and activated carbon for adsorbing radioactive iodine. CLEAN–Soil Air Water 39(2): 103–108
Wang J, Ai K, Lu L (2019) Flame-retardant porous hexagonal boron nitride for safe and effective radioactive iodine capture. J Mater Chem A 7:16850–16858
Li H, Li Y, Li B, Liu D, Zhou Y (2020) Highly selective anchoring silver nanoclusters on MOF/SOF heterostructured framework for efficient adsorption of radioactive iodine from aqueous solution. Chemosphere 252:126448
Yang RT (2003) Adsorbents: fundamentals and applications. John Wiley, USA
Huve J, Ryzhikov A, Nouali H, Lalia V, Augé G, Daou TJ (2018) Porous sorbents for the capture of radioactive iodine compounds: a review. RSC Adv 8:29248–29273
Wu L, Sawada JA, Kuznicki DB, Kuznicki T, Kuznicki SM (2014) Iodine adsorption on silver-exchanged titania-derived adsorbents. J Radioanal Nucl Chem 302:527–532
Azambre B, Chebbi M, Hijazi A (2020) Effects of the cation and Si/Al ratio on CH3I adsorption by faujasite zeolites. Chem Eng J 329:12308
Cheng Q, Yang W, Li Z, Zhu Q, Chu T, He D, Fang C (2015) Adsorption of gaseous radioactive iodine by Ag/13X zeolite at high temperatures. J Radioanal Nucl Chem 303:1883–1889. https://doi.org/10.1007/s10967-014-3736-3
Rastunov LN, Magomedbekov EP, Obruchikov AV, Lomazova LA (2011) Evaluation of the sorbent layer thickness in iodine filters. At Energ 110:68–72. https://doi.org/10.1007/s10512-011-9392-6
Thomas T, Staples B, Murphy L, Nichols J (1977) Airborne elemental iodine loading capacities of metal zeolites and a method for recycling silver zeolite. Allied Chemical Corp., Idaho Falls, Idaho (USA). Idaho Chemical Programs …,
Liu Y, Wei G, Feng Y, Lu X, Chen Y, Sun R, Peng L, Ma M, Zhang Y, Zhang Z (2020) The effect of boron on zeolite-4A immobilization of iodine waste forms with a novel preparation method. J Radioanal Nucl Chem 324:579–587. https://doi.org/10.1007/s10967-020-07079-8
Chebbi M, Azambre B, Cantrel L, Huvé M, Albiol T (2017) Influence of structural, textural and chemical parameters of silver zeolites on the retention of methyl iodide. Microporous Mesoporous Mater 244:137–150. https://doi.org/10.1016/j.micromeso.2017.02.056
Bruffey SH, Jubin RT, Patton KK, Walker JF Jr (2014) Aging of iodine-loaded silver mordenite in NO2. Oak Ridge National Lab.(ORNL). Oak Ridge, TN (United States)
Soelberg N, Watson T (2012) Iodine sorbent performance in FY 2012 deep bed tests. Idaho National Lab.(INL). Idaho Falls, ID (United States),
Belapurkar A, Rao KA, Gupta N, Iyer R (1984) On the interaction of CH3I over silver-exchanged molecular sieves. Surf Technol 21:263–272
Park G-I, Kim I-T, Lee J-K, Ryu S-K, Kim J-H (2001) Effect of temperature on the adsorption and desorption characteristics of methyl iodide over TEDA-impregnated activated carbon. Carbon Lett 2:9–14
Holladay D (1979) Literature survey: methods for the removal of iodine species from off-gases and liquid waste streams of nuclear power and nuclear fuel reprocessing plants, with emphasis on solid sorbents
Acknowledgements
This research was supported by the National Natural Science Foundation of China under Grant Agreement No. U1967215. This work was also supported by the “Reprocessing Scientific Research Special Program”, which is managed by the National Defense Bureau of Science and Industry under Grant Agreement No. KY20007.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, J., Chen, X., Ren, H. et al. Study on adsorption characteristics of radioactive gaseous iodine on Ag ion exchange molecular sieve. J Radioanal Nucl Chem 332, 2269–2277 (2023). https://doi.org/10.1007/s10967-023-08826-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-08826-3