Abstract
Aiming at radioactive cesium waste treatment, this work report a low-temperature immobilization method to convert Cs-polluted waste into pollucite. Results reveal that pollucite can be synthesized over a wide range of Cs content (36.5–53.2 wt.%) at 500 °C via the CsOH-Zeolite 4A-Silica gel system. The synthesized pollucite is composed of lamellar particles (0.8–2.5 μm) along with the agglomerates of small particles (0.1–0.3 μm) and Cs, Al, Si, O elements distribute uniformly on surface. Cs content and chemical stability analysis indicate 99.24% of Cs are immobilized and the leaching rate of Cs is on the order of 10–3 g/(m2 ·d).
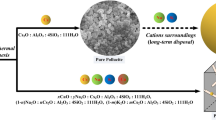
Graphical abstract












Similar content being viewed by others
References
Jianda J, Tao Y (2019) Enrichment and ecological risk of cesium-137 in marine organisms in the waters adjacent to coastal nuclear power stations in China. Asian J Ecotoxicol 14(1): 67–74. 10.7524/AJE.1673–5897.20181101002
Huiling W (2012) Cesium-137 deposition and contamination of Japanese soils due to the Fukushima nuclear accident. Chin J Agric Biotechol 20(3):315
Haoran C, Xiaobin Z, Jiasheng R (2017) Removal of cesium from highly contaminated water in Fukushima Daiichi nuclear power station. Shandong Chem Ind 46(7):186–188
I.W. D, B.L. M, R.N.J. T, (1997) The immobilization of high level radioactive wastes using ceramics and glasses. J Mater Sci 32:5851–5887. https://doi.org/10.1023/A:1018646507438
Gabriele M, Rossella A, Giovanna V et al (2011) Borosilicate and aluminosilicate pollucite nanocrystals for the storage of radionuclides. Powder Technol 208(2):491–495. https://doi.org/10.1016/j.powtec.2010.08.048
D.M. S, W.W. S, (1977) Characterization of pollucite as a material for the long term storage of cesium-137. Atlantic Richfield Hanford Co., Richland, WA (USA)
Richard MB (1969) The crystal structure and chemical composition of pollucite. Z Kristallogr Cryst Mater 129:280–302. https://doi.org/10.1524/zkri.1969.129.16.280
W.H. T, (1938) Note on the structures of analcite and pollucite. Z Kristallogr Cryst Mater 99(1):283–290. https://doi.org/10.1524/zkri.1938.99.1.283
Natsumi K, Koji N, Yoshinobu Y (2008) Crystal structure of pollucite. Z Kristallogr 223:584–590. https://doi.org/10.1524/zkri.2008.1103
P. B, D. C, B. L, et al (2004) Safe trapping of Cs in heat-treated zeolite matrices. J Nucl Mater 324(2–3):183–188. https://doi.org/10.1016/j.jnucmat.2003.10.001
Luis HO, Michael DK, Sean MM (2010) Pollucite and feldspar formation in sintered bentonite for nuclear waste immobilization. Appl Clay Sci 50:594–599. https://doi.org/10.1016/j.clay.2010.10.003
Ian M, Jesus C, Clive BP (1999) Hydrothermal synthesis of pollucite (CsAlSi2O6) powders. J Am Ceram Soc 82(11):3242–3244. https://doi.org/10.1111/j.1151-2916.1999.tb02231.x
Mia O, Ljiljana M, Jovana R et al (2016) Safe trapping of cesium into pollucite structure by hot-pressing method. J Nucl Mater 474:35–44. https://doi.org/10.1016/j.jnucmat.2016.03.006
Zhenzi J, Wenbo H, Xiaojun H et al (2016) A novel hydrothermal method to convert incineration ash into pollucite for the immobilization of a simulant radioactive cesium. J Hazard Mater 306:220–229. https://doi.org/10.1016/j.jhazmat.2015.12.024
Yuqian C, Zhenzi J, Kunchuan C et al (2018) Hydrothermal conversion of Cs-polluted soil into pollucite for Cs immobilization. Chem Eng J 336:503–509. https://doi.org/10.1016/j.cej.2017.11.187
Zhenzi J, Kunchuan C, Yan L et al (2017) Hydrothermal synthesis of pollucite, analcime and their solid solutions and analysis of their properties. J Nucl Mater 488:63–69. https://doi.org/10.1016/j.jnucmat.2017.03.008
Junjie F, Zhenzi J, Yi Z et al (2016) Mild hydrothermal synthesis of pollucite from soil for immobilization of Cs in situ and its characterization. Chem Eng J 304:344–350. https://doi.org/10.1016/j.cej.2016.06.077
Zhenzi J, Yuan Y, Wenbo H et al (2018) Synthesis of pollucite with Cs-polluted incineration ash mixed with soil for immobilization of radioactive Cs. J Nucl Mater 510:141–148. https://doi.org/10.1016/j.jnucmat.2018.07.047
Peigang H, Shuai H, Meng W et al. (2020) B2O3-assisted low-temperature crystallization of pollucite structures and their potential applications in Cs+ immobilization. J Nucl Mater 540:152314. https://doi.org/10.1016/j.jnucmat.2020.152314
Lei L, Zhonghui X, Han L et al (2022) Immobilization of strontium and cesium by aluminosilicate ceramics derived from metakaolin geopolymer-zeolite A composites via 1100 °C heating treatment. Ceram Int 48(11):15236–15242. https://doi.org/10.1016/j.ceramint.2022.02.054
Peigang H, Ruifei W, Shuai F et al (2019) Safe trapping of cesium into doping-enhanced pollucite structure by geopolymer precursor technique. J Hazard Mater 367:577–588. https://doi.org/10.1016/j.jhazmat.2019.01.013
Guilin W, Bingsheng L, Zhentao Z, et al. (2019) Boron assisted low temperature immobilization of iodine adsorbed by silver-coated silica gel. J Nucl Mater 526:151758. https://doi.org/10.1016/j.jnucmat.2019.151758
Guilin W, Xiaoyan S, Zhentao Z et al. (2020) B2O3–Bi2O3–ZnO based materials for low-sintering temperature immobilization of iodine adsorbed waste. J Solid State Chem 289:121518. https://doi.org/10.1016/j.jssc.2020.121518
Yi L, Yaxin Feng, Guilin W et al. (2021) Synthesis of glass composite material with bismuthate glass powder and zeolite-4A for immobilization of iodine waste. J Solid State Chem 294:121856. https://doi.org/10.1016/j.jssc.2020.121856
Yi L, Guilin W, Yaxin F et al (2020) The effect of boron on zeolite-4A immobilization of iodine waste forms with a novel preparation method. J Radioanal Nucl Chem 324:579–587. https://doi.org/10.1007/s10967-020-07079-8
Yaxin F, Guilin W, Yi L et al. (2022) Crystallization behavior of boron in low-temperature immobilization of iodine waste. J Solid State Chem 305:122698. https://doi.org/10.1016/j.jssc.2021.122698
Yaxin F, Guilin W, Zhentao Z et al (2022) Immobilization of iodine waste via the gas-pressure sintering of glass-bonded iodosodalite ceramic. Ceram Int 48:16312–16318. https://doi.org/10.1016/j.ceramint.2022.02.181
Yoshinobu Y, Satoru I (1998) The crystal structure of analcime. Micropor Mesopor Mater 21(4–6):365–370. https://doi.org/10.1016/S1387-1811(98)00019-5
Seyed Naser A, Akram Alavi D, Maryam A (2013) Phase transformation of zeolite P to Y and Analcime Zeolites due to changing the time and temperature. J Spectrosc 2013:428216. https://doi.org/10.1155/2013/428216
Carmen S, Chi-Hong C, G. Diego G (2020) Single-crystal elastic properties of (Cs,Na)AlSi2O6·H2O pollucite: a zeolite with potential use for long-term storage of Cs radioisotopes. J Appl Phys 108:093509. https://doi.org/10.1063/1.3504613
Saehwa C, Jacob AP, Brian JR et al (2018) Glass-bonded iodosodalite waste form for immobilization of 129I. J Nucl Mater 504:109–121. https://doi.org/10.1016/j.jnucmat.2018.03.033
Klima KM, Schollbach K, Brouwers HJH, Yu Q (2022)Enhancing the thermal performance of Class F fly ash-based geopolymer by sodalite. Conster Build Mater 314:125574. https://doi.org/10.1016/j.conbuildmat.2021.125574
Dumitru Doru Burduhos N, Mohd Mustafa Al Bakri A, Andrei Victor S et al. (2020) XRD and TG- DTA study of new alkali activated materials based on fly ash with sand and glass powder. Materials. 13(2):343. https://doi.org/10.3390/ma13020343
Qin L, Hui X, Feihu L et al (2012) Synthesis of geopolymer composites from blends of CFBC fly and bottom ashes. Fuel 97:366–372. https://doi.org/10.1016/j.fuel.2012.02.059
Acknowledgements
The work reported here was supported by the Fundamental Science on Nuclear Wastes and Environmental Safety Laboratory (No. 19kfhk02).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Feng, Y., Wei, G., Liu, Y. et al. Synthesis and characterization of pollucite: a low-temperature immobilization method for 137Cs. J Radioanal Nucl Chem 332, 467–478 (2023). https://doi.org/10.1007/s10967-022-08736-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-022-08736-w