Abstract
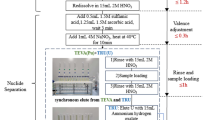
The plutonium rapid response in vitro bioassay method described here was designed to determine 238Pu, 239Pu, and 240Pu concentration in urine samples from workers with potential internal contamination. Results provide quick and actionable information about the level of contamination necessary to assist making further medical decisions, including chelation therapy. The radiochemical procedure can be performed within approximately 48 h, including sample preparation, measurement(s) by alpha spectrometry and/or inductively coupled plasma mass spectrometry (ICP-MS), and data evaluation.

Similar content being viewed by others
References
Klumpp JA Bioassay Monitoring at Los Alamos National Laboratory – booklet - LA-UR-18-25219
Kumar R, Rao DD, Dubla R, Yadav JR (2017) A rapid method for estimation of Pu-isotopes in urine samples using high volume centrifuge. Appl Radiat and Isotopes 125:176–179
Ni Y, Zheng J, Guo Q, Men W, Tagami K, Uchida S (2018) Rapid determination of ultra-trace plutonium isotopes (239Pu, 240Pu and 241Pu) in small-volume human urine bioassay using sector-field inductively coupled plasma mass spectrometry. Anal Chim Acta 1000:85–92
Hang W, Zhu L, Zhong W, Mahan C (2004) Separation of actinides at ultra-trace level from urine matrix using extraction chromatography-inductively coupled plasma mass spectrometry. J Anal At Spectrom 19:966–972
Dulanska S, Bilohuscin J, Remenec B, Galanda D, Matel L (2015) Determination of 239Pu, 241Am and 90Sr in urine using pre-filter material and combined sorbents AnaLig Pu-02, AnaLig Sr-01, DGA Resin J. Radioanal Nucl Chem 304:127–132
Hernandez Gonzales C, Bercedo IS (2019) Rapid procedure for actinides and 90Sr analysis in emergency urine spot samples applied in the GHSI-RNWG emergency intercomparison exercise. Appl Radiat and Isotopes 144:19–23
Maxwell SL, Jones VD (2009) Rapid determination of actinides in urine by inductively coupled plasma mass spectrometry and alpha spectrometry: A hybrid approach. Talanta 80:143–150
Maxwell SL, Culligan BK (2009) New column separation method for emergency urine samples. J Radioanal Nucl Chem 279 1:105–111
Zagyvai M, Vajda N, Groska J, Molnar Zs, Bokori E, Szeredy P (2017) Assay of actinides in human urine by rapid method. J Radioanal Nucl Chem 314:49–58
Maxwell SL (2008) Rapid analysis of emergency urine and water samples. J Radioanal Nucl Chem 275 3:497–502
Vasile M, Jacobs K, Bruggeman M, Van Hoecke K, Dobney A, Verrezen F (2018) On the sequential separation and quantification of 237Np, 241Am, thorium, plutonium, and uranium isotopes in environmental and urine samples. Appl Radiat and Isotopes 134:455–460
Dai X, Kramer-Tremblay S (2011) An Emergency Bioassay Method for Actinides in Urine. Health Phys 101 2:144–147
Eppich GR, Macsik Z, Katona R, Konegger-Kappel S, Stadelmann G, Koepf A, Varga B, Boulyga S (2019) Plutonium assay and isotopic composition measurements in nuclear safeguards samples by inductively coupled plasma mass spectrometry. J Anal At Spectrom 34:1154–1165
Harris MN, Hudston LA, Macsik Z, Lopez DM, Eaton SJ, Zazueta JA, Zuniga MM, DeBacker KB, Baca J, Margiotta C, Inglis JD, LaMont SP, Steiner, R E (2022) A Method for Identifying and Re-establishing Equilibrium Between Tracer and Analyte Within Plutonium Bioassay. International Conference on Methods and Applications of Radioanalytical Chemistry (MARC), 2022-04-02/2022-04-09 Kona, Hawaii, United States
Begley IA, Sharp BL (1997) Characterisation and Correction of Instrumental Bias in Inductively Coupled Plasma Quadrupole Mass Spectrometry for Accurate Measurement of Lead Isotope Ratios. J Anal At Spectrom 12:395–402
Curie LA (1968) Limits for qualitative detection and quantitative determination. Application to radiochemistry. Anal Chem 40 3:586–593
Gerber GB, Thomas RG(1992) Guidebook for the treatment of accidental internal radionuclide contamination of workers.Radiat. Protect Dosim.41(1)
U.S. Department of Energy (2013) Good practices for occupational radiological protection in plutonium facilities. USDOE; DOE-STD-1128‐2013, Washington, DC
Los Alamos National Laboratory. Responding to Radiological Incidents, RP-SVS-ID-DP-06.DATS
Glover L, Bertelli L, Dumit S, Poudel D, Smith L, Waters T, Klumpp J(2022) Side effects and complications associated with treating plutonium intakes: a retrospective review of the medical records of LANL employees treated for plutonium intakes, with supplementary interviews. Health Phys. Published Ahead of Print
International Commission on Radiological Protection (1993) Age dependent doses to the members of the public from intakes of radionuclides. Pergamon Press, New York. ICRP Publication 67; Ann. ICRP 23(3/4)
Miller G (2010) Biokinetic models for plutonium. Los Alamos. Los Alamos National Laboratory, NM. Radiological Dose Assessment Technical Issue Paper 003
Fleischer RL, Raabe OG. Fragmentation of respirable PuO2 particles in water by alpha-decay: a mode of ‘dissolution’(1978) Solid State Nuclear Track Detectors ed F Grancer, H Patezke and E Schopper. Oxford, UK: 663–668 2d4. Diel J H, Mewhinney J A. Breakup of inhaled 238PuO2 particles in the lungs of hamsters and dogs. (1980) Inhalation Toxicology Research Institute Annual Report 1979-90, LMF-84; Albuquerque, NM 17–19
National Council on Radiation Protection and Measurements (2006) Development of a biokinetic model for radionuclide contaminated wounds and procedures for their assessment, dosimetry and treatment. NCRP, Bethesda, MD. Report No. 156
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Macsik, Z., LaMont, S.P., Cardon, A.M.R. et al. Rapid response in vitro bioassay method for the determination of Pu isotopes in urine samples. J Radioanal Nucl Chem 331, 5359–5369 (2022). https://doi.org/10.1007/s10967-022-08616-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-022-08616-3