Abstract
Three different dosimetry systems were prepared based on diphenyl thiocarbazone (DTH) dye: polymer films made with, poly(vinyl alcohol), DTH liquid dosimeter and DTH gel dosimeter. All the systems received several gamma doses. The three systems were analyzing by UV–Vis spectrophotometery before and after irradiation. The effective dose range in the linear part of these systems is between 1–10 kGy, 0.3–2 kGy, and 50–200 Gy. The dose range of these three dosimeters relies on the concentration of DTH. The radiation chemical yield (G-value) was determined for the three prepared dosimeters, and all three radiation dosimeters were examined before and after irradiation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Applications of ionizing radiation are numerous in the scientific, medical, and industrial sectors [1]. Gamma irradiation monitoring is also essential in irradiation facilities and a variety of other applications [2]. The science of dosimetry is the measurement of doses [3]. When the change in the material produced and subjected to gamma rays is discernible, regular, quantifiable, and stable over time, dosimeters are used [4]. During the duration of the period, numerous types of dosimeters are utilized for a variety of purposes, including dyed polymeric thin plastic film dosimeters, which have been widely applied [5,6,7,8,9,10,11]. Liquid dosimeters [12,13,14,15,16,17,18], gel dosimeters [19,20,21,22] and EPR dosimeters [23,24,25]. Upon exposure to irradiation, the physical and chemical properties of the polymers change in the case of films, accordingly due to induced cross-links, chain detachments and formation of new chemical bonds [26]. Previous researches have primarily focused on the effect of radiation on the liquid which causes the formation of temporary active species such as free radicals, free ions and excited molecules, which can then be monitored by using optical spectrum, electron spin resonance spectra, conductivity detection or some other suitable techniques [13]. In addition to, color bleaching of organic dyes induced by γ-rays has been disscussed and applied [22, 27], that Color bleaching might be investigated in phrases of H. and.OH radical’s interaction with the dyes in aqueous solutions [28].
The existing work aimed to prepare, investigate current dosimetry systems of Diphenyl thiocarbazone as films, liquid and gel dosimetry systems and research the spectrophotometric response of the three dosimeters irradiated with gamma rays. The dose–response function, radiation sensitivity, and dependences of the response to environmental factors have been studied.
Experimental procedures
Preparation of diphenyl thiocarbazone dosimetry systems
Preparation of stock solution of diphenyl thiocarbazone (DTH)
The stock solution of DTH dye was first prepared by dissolving 0.08 g (DTH) in 50 mL ethanol (6.24 × 10–3 Molar). The liquid was once stirred at room temperature for 24 h to obtain a complete dissolution of all dye.
Preparation of DTH/PVA film dosimeter
DTH/PVA films had been prepared by dissolving 5 g of poly(vinyl alcohol) PVA(fully hydrolyzed 99–100% average) common molecular weight 125,000 daltons (product of Wacker Co., USA), in a 100 mL of double-distilled water at approximately 60 °C [5]. The stock solution of the polymer was stirred at this temperature for about 24 h and left to cool. Then, it was divided into eight groups, with 13 mL each of PVA solution and 1.25, 1.8, and 2.5 mL of dye stock solution was added and stirred for approximately three hours at room temperature to acquire a uniformly dyed solution. Moreover, DTH (from Sigma Aldrich, USA) has the molecular weight 256.33 g/mol was used to prepare three different concentrations (1.5 × 10–4, 2.2 × 10–4, and 3.12 × 10–4 mmolL−1 equivalent to 0.31, 0.46, and 0.62 phr, where phr* = part per hundred parts of resin). The dyed polymer solution was once stirred, put in a Petry dish and left to dry at room temperature for approximately forty eight hours. The film thickness measured using a Digitrix-Mark II thickness gauge (precision ± 1 µm, 1σ), its thickness was 0.039 ± 0.005 mm.
Preparation of (DTH-liquid) dosimeter
Three specific concentrations of the dye (0.1498, 0.225, and 0.281 mmolL−1) were prepared by adding (1.25, 1.8, and 2.5 mL) of the stock solution of DTH.
Preparation of (DTH-gelatin) gel dosimeter
Three distinct concentrations of dye from the stock solution were used (0.137, 0.196, and 0.275 mmolL−1 or 1.1, 1.65, and 2.2 mL) and mix with a gelatin twenty percent w/w (i.e., the quality of gelatin is related to the quality of the final gel, we know that the clarity of well-prepared gelatin gel samples depends on the dissolving of all portions of gelatin in water, and that we chose this type of gelatin based on past research [16, 22].). Gelatin from porcine skin (300 blooms, G2500, Sigma-Aldrich) was dissolved in distilled water, and then the DTH dye C6H5NHNHCSN = NC6H5 was introduced from the stock solution. The liquid combination was constantly stirred in the water bath, forming a yellow color. Because the response depends on temperature, the water bath was kept at 70 ± 5 °C for four hours (That the temperature has two main causes: first, the viscosity of gelatin, which is not better after this temperature; second, the colour of the dye incorporating with the gelatin, which causes colour bleaching of the dye, which affects the sample doses response). This temperature was chosen for a quick coloration. The samples were pipetted into a 1 cm thick glass test tube and kept in a fridge close to 4 °C for 4 h. Scheme 1 shows the chemical structure of Diphenyl thiocarbazone as shown in the following structure.

Analysis
The UVIKON 860 spectrophotometer (Unicam Co. Ltd, England) was used to report the absorption spectra of unirradiated and irradiated dyed at wavelength variation of 200–800 nm (The films are analysed by cutting the film sheet into 1 cm × 1 cm pieces, measuring all of them before irradiation, and taking each film exposed to specified absorbed doses at gamma irradiation facilities. After exposing to different absorbed doses, these films were measured with the spectrophotometer. We measured 4 sample films for each dose. Finally, we chose the gradually decreasing shape of these irradiated films. Spectral UV/VIS measurements were undertaken at the selected λmax wavelength (nm) for each film before and after irradiation. The average response curve of each four films were plotted ΔA/Ao against absorbed dose (kGy).
Irradiation facility
The irradiation was performed with the Co-60 gamma chamber 4000A irradiation device (at the Bhabha Atomic Research Center, India) with an absorbed dose rate of 1.22 kGy h−1. The dose rate of the gamma source was measured using a ferrous sulfate (Fricke dosimeter) [29].
Pre-irradiation stability
By keeping the films, solutions, and gel samples at an enclosed temperature (25 °C ± 2) in the dark and under fluorescent laboratory lights, the color stability of the three dosimetry systems DTH/PVA films, DTH/solutions, and DTH/gelatin was improved. All of these samples had their absorbance measured at 537, 526, and 530 nm; respectively. Throughout the pre-irradiation storage period, for a total of 63 days (for films and solutions, and around 36 days for gel samples). The change in absorption was measured.
Post-irradiation stability
DTH/PVA films, DTH/solutions, and DTH/Gelatin samples irradiated at 5 kGy, 1 kGy, and 100 Gy; respectively, and stored at room temperature (25 °C ± 2) in the dark and under fluorescent light laboratory. The absorbance of these irradiated samples was measured at a wavelength of 537, 526, and 530 nm at different times within 63 days during the post-irradiation storage period (for films and solutions) and 36 days for gel samples. The change in the absorbance a function of storage time and pre-storage measured (immediately after irradiation).
Results and discussion
Absorption spectra
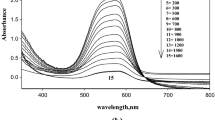
To acquire a good dosimeter in any form, as a thin plastic film, a solution, or a gel dosimeter, the change can be consistent, regularly, and with good stability [3]. The optical dentistry DTH/PVA (0.62 phr, parts per hundred parts of resin) before and after irradiation was recorded at various doses, as indicated in Fig. 1a. The unirradiated and irradiated DTH/PVA film absorption spectrum shows a sharp band in the visible light region, reaching its maximum at 537 nm. Although the pink color is dependent on the wavelength, this maximum corresponds to the electronic excitation of the dye described in the text. The intensity of the absorption band decreases steadily as the dose of γ-rays increases (That is, flexible plastic films change from pink to colorless). These films, before irradiation, were elastic; but after irradiation, they become more fragile, increasing the fragility by increasing absorbed doses. This means that bleaching has been held regularly during exposing gamma-radiation. On the other hand, the optical density of irradiated and unirradiated DTH solution (0.281 mmolL−1 of the dye) was reported to distinctive doses as proven in Fig. 1b. The absorption spectrum of unirradiated solutions shows an absorbance peak in the visible region at 526 nm. The absorption peak gradually decreases with increasing the absorbed dose. However, as shown in Fig. 1c, the absorption spectra of the unirradiated and irradiated samples imply an absorption peak in the observed region for liquid gel polymer at 530 nm (typical of a pink color). This suggests that the density of all absorption peaks in the seen spectra is step by step reduced by the increase of gamma-radiation.
a. The absorption spectra of the unirradiated and gamma-irradiated DTH/PVA films to different absorbed doses, [DTH] = 0.62 phr. b. The absorption spectra of the unirradiated and gamma-irradiated DTH solutions to different absorbed doses, [DTH] = 0.281 mmolL-1. c. The absorption spectra of unirradiated and gamma-irradiated (DTH– gelatin) gel to different absorbed doses, [DTH] = 0.275 mmolL-1
Dose–response curves
For dosimetry, optical density was monitored at 537 nm for DTH/films with 0.62 phr DTH. Figure 2a. Suggests that the response curves of DTH /PVA films containing distinctive concentrations of the dye (0.31, 0.46 and 0.62 phr, where phr* = part per hundred parts of resin), ΔA.mm−1 at 537 nm, versus the absorbed dose, D, in which (ΔA = Ao − Ai) wherein Ao and Ai are the values of the absorption coefficient for the unirradiated and irradiated films; respectively. The recorded doses interval is from 1 to 110 kGy. The usable dose with linear behavior from the response curve is from 1 to 10 kGy and relies on the dye concentration. Likewise, this is similar to the other systems. Figure 2b. Indicates the response curves of DTH liquid include various DTH concentrations (0.1498, 0.225, and 0.281 mmol L−1). These curves were established in terms of change in optical density at 526 nm against the absorbed dose (∆A = Ao − Ai), when Ao and Ai are values of optical density for the unirradiated and irradiated solutions; respectively. The curves display that the dose variety extends up to 10 kGy, Fig. 2b. Disclose the linear partial reaction of the DTH liquid at 526 nm. The dose dependencies are linear up to 2 kGy. On the other hand, the group of gel samples has been irradiated inside the range amongst 50 and 2200 Gy. Over that dose, the system has a tendency to saturate. The ensuing gel color has an absorbance peak at 530 nm, this peak height decreased upon irradiation with γ-rays because the gel degraded. Figure 2c. Displays the reaction curves of the dyed-gel pattern containing various DTH concentrations. Every dose corresponds to a 4 replicated check tube pattern (This means not dealing with various varities of gelatin, but it means that each dose on the response curve was used in 4 test tubes for the same dyed gelatin mixture). The linear correlation coefficients have been calculated to be 0.99891, 0.99978, and 0.999887 for the prepared composite (dyed gel) concentrations 0.137, 0.195 and 0.275 mmol L−1 of DTH dye; respectively. Furthermore, all doses that can change the color of the dye to be bleached are taken from Figs. 2a–c; 3a–c are the figures that explain the linear dose sensitivity to gamma rays, which in the case of film dosimeters is very sensitive in the liner part to -radiation in the range of 1–10 kGy, in solution dosimeters the effective dose range was 0.3–2 kGy, and the effective dose range of gel dosimeters was 50–200 Gy.
a. Change of ΔA, as a function of absorbed dose for different DTH concentrations in PVA films. [DTH] = 0.62 phr or 3.12 × 10–4. b. Change of ΔA, at 526 nm, as a function of absorbed dose for different DTH concentrations in solution, [DTH] = 0.281 mmolL-1. c. Response curves of the (DTH– gelatin) gel at 530 nm in the dose range of 50–2200 Gy, [DTH] = 0.275 mmolL-1
Radiation sensitivity of three different dosimetry systems prepared
The discussion of each of the alternative three dosimetry systems are greater significant to the γ-rays based not only on the dose range but also the values that can be estimated from the available equation that sensitivity = the slope of the three-dimensional linear regression of response curves from Fig. (3a–c) to ΔA/Dose for each curve [2]. By applying this equation in one concentration of each film, the solution, and gel dosimetry system, there is a greater sensitivity or effect on gel dosimeters more than in the solutions, as well as in the film i.e. the gel dosimeters are the most sensitive dosimeters. All dosimeters composed of thin plastic films, such as PVA, can be categorized as high-dose dosimetry applications, beginning with the first device composed of DTH and PVA [6,7,8], the rigidity and free reactive species (H. And OH.) released from the structure of the polymer increasing with gamma absorbed doses. However, the second system relies on the same dye dissolved in ethanol, which have many active species OH., e−eq. H., H2, H2O2, H3O+aq, H+, OH−) [27]. Similarily, in the case of the third gel dosimetry system, it relies on the identical material DTH incorporated into gelatin dissolved in water, but the reason for its higher sensitivity compared with the previous system films and solutions is based on the following hypothesis: Gelatin will be affected by γ-rays on its structure, so [20, 22] explained its effective role in fasting bleaching process. Gelatin is a polypeptide with a shape wherein more than one amino acids are connected through peptide bonds, (–NH–CO–), that gelatin is involved in the dosimeter’s response. The amino group inside the dosimeter liquid plays a major role in the free radical degradation in DTH-gel dosimeters.
Radiation chemical yield (G-value)
With the average value of the dye concentration in mol/L and A0/b (in which b is the slope of the linear portion of the dose–response curve), the molar extinction coefficient is estimated to be 39,622.3 L.mol−1 cm−1 DTH/PVA films. The density of the polymer (1.25 g. cm−3) is used to estimate the G-value in terms of µmol/J. The calculated G-value of these films and the concentration of the dye within the film component are listed in the Table (1). Moreover, taking the dye concentration in mol/L as the unit and taking the average value of A0/b, the molar extinction coefficient is calculated to be 6841.058 L.mol−1 cm−1 DTH solution and 1926 L.mol−1 cm−1 DTH in the gel. On the other hand, it can be seen from the table that the G-value of each medium increases with the increase of the dye concentration, and the number of moles of the dye is degraded by absorbing of 1 J of energy (unit: mol/J) [5, 30]. The radiation chemical yield (G-Value) in the gel dosimetry system is higher than that of the solution than that of thin plastic film, indicating that the gel dose measurement method is more sensitive than the solutions and film dosimetry systems [22]. According to these findings, DTH in the form of a gel is more sensitive to gamma-radiation and low-dose measurement processing, such as radiotherapy, seed manufacture, and blood irradiation. The mild sensitivity of DTH as a solution, on the other hand, reflects its use in food irradiation processing. Finally, DTH as a thin plastic film is less sensitive to gamma rays or high-dose dosimetry applications than gels and solutions, reflecting its uses in polymer degradation and cross-linking, as well as medical sterilizing [1, 3].
Suggested mechanism for the dye bleaching after irradiation
It is apparent that the dye has a conjugated system based on its structure. The effect of gamma rays after irradiation is non-selective, and it can destroy the dye's structure, including the active azo group –N = N- and the –C = S-group in the structure. As the absorbed doses increase, all conjugated or chromophores units as –C = C– that exist in the structure of DTH dye are destroyed. This is the fundamental reason why dye becomes bleached and loses its color after being exposed to gamma radiation. This can be expressed using the following colors, which decrease in intensity from pink to light pink to very light pink and finally to colorless.
Pre-irradiation stability
The color balance of the DTH/PVA film (0.62 phr) was modified by keeping the film at an enclosure temperature (25 °C ± 2) in the darkish and beneath fluorescent laboratory lights. The absorbance of these films was measured at 537 nm during specified storage period of 63 days throughout the pre-irradiation storage period. The exchange in absorbance of 537 nm as a role of hold period and pre-storage (right away behind stripping) is proven in Fig. 4a. The films can be considered to show a slight decrease in absorbance at the same time of the first 5 days after the film has finally become stable until the end of shelf life under two storage conditions of storage (dark & light). Moreover, to assess the potential products of before irradiation on the organized liquid samples, we examined the absorbance of un-irradiated solution samples stored under certain conditions. Liquid samples that we stored at room temperature exposed to the light fluorescent laboratory and dark as shown in Fig. 4b. And their absorbencies at 526 nm, which we analyzed for 63 days. Remained unchanged during the test period. Excluding the first 10 days from the change or storage time, then it is desirable until the end of the storage period. Alternatively, to evaluate the potential safety pre-irradiation effects on the prepared gel samples, we monitored the absorbance of the un-irradiated gel sample stored under different conditions. Two groups of gel samples that we restored under different conditions and their absorbencies at 530 nm we have monitored for 36 days. One group was stored in the dark at 4 °C, and the opposite group become saved at an enclosure temperature uncovered to laboratory fluorescent mild as it is presented in Fig. 4c. The absorbencies of the samples saved stayed basically without change at some point of the whole length of the experiment. Except for the first 7 days from the fading or storage time, then assume that it is regular till the give up of the storage length.
a. Pre-irradiation stability of DTH/PVA films stored in direct and indirect light at room temperature, as a function of storage time (Error bars on this Fig. is so small). b. Pre-irradiation stability of DTH solutions stored in direct and indirect light at room temperature, as a function of storage time. (Error bars on this Fig. is so small). c. Pre -irradiation stability of (DTH–gelatin) gels stored under different storage conditions (Dark and light). [DTH] = 0.275 mmolL-1. (Error bars on this Fig. is so small)
Post-irradiation stability
DTH/PVA film contains [DTH] = 0.62 phr irradiated at 5 kGy, stored at room temperature (25 °C ± 2) in the dark and under fluorescent light laboratory. The absorbance of these films was measured at a wavelength of 537 nm at different times within 63 days during the post-irradiation storage period. The change in the absorbance at 537 nm as a function of storage time and pre-storage (immediately after stripping) is shown in Fig. 5a. From this figure, it can be seen that the irradiated films show good stability under storage conditions. In addition, the DTH solutions contained [DTH] = 281 µmolL−1 irradiated at 1 kGy, stored at room temperature (25 °C ± 2) in the dark and under fluorescent light laboratory. The absorbance of these solutions was measured at a wavelength of 526 nm at different times within the 63 day post-irradiation storage period. The change in absorbance at 526 nm as a function of storage time, compared with that before storage (immediately after irradiation), as shown in Fig. 5b. From this figure, it can be seen that the irradiated solutions show good stability under storage conditions. With the exception of the first 11 days, it gradually decreases, becoming more stable until the end of the storage period. Finally, most of the gels (DTH–gelatin) had been exposed to γ-rays with 100 Gy. Behind the irradiation, they were kept in various cases. A set become saved in an enclosure temperature beneath the laboratory fluorescence light; another one was saved at 4 °C in the darkish. The absorbencies of the sets at 530 nm were estimated regularly over a period of 36 days of storage, that gelatine is not dehydrated as shown in Fig. 5c. The relative absorbancies of the samples saved at 4 °C stayed basically without change at some point of the whole length of the experiment.
a Post-irradiation stability of DTH/PVA films stored in direct and indirect light at room temperature, as a function of storage time, at 5 kGy. (Error bars on this Fig. is so small). b. Post-irradiation stability of DTH solutions stored in direct and indirect light at room temperature, as a function of storage time, at 1 kGy. (Error bars on this Fig. is so small). c. Post-irradiation stability of (DTH–gelatin) gels stored under different storage conditions (irradiated at 100 Gy). (Error bars on this Fig. is so small)
Comparative study of three dosimetry systems based on the same material but different media and solvents.
Through studying the records in Table 2, it is possible to determine whether Diphenyl thiocarbazone (DTH) dye is used as a completely sensitive material in three different media as film, liquid and gel systems makes it sensitive material to γ-rays and may be carried out in lots of fields of gamma-irradiation processing as radiation dosimeters. On the other hand, there are many dosimeters that have a low dose range, but has higher sensitivity [22, 23], and there are films-based systems that have a lower sensitivity [8, 9], there are systems solutions-based systems as moderate sensitivity [12, 18]. Similarly, this study indicates that the DTH/film system is sensitive to gamma-rays but its sensitivity is lower than DTH solutions and the most sensitive one towards γ-rays was the gel dosimeter.
Conclusion
Three different dosimetry systems were prepared based on Diphenyl thiocarbazone (DTH) dye: polymer films made with, poly (vinyl alcohol) (PVA), DTH liquid dosimeter and DTH gel dosimeter. All the systems received several gamma doses of irradiation. The three systems were analyzing by UV–Vis spectrophotometery before and after irradiation. DTH/PVA film shows a high absorption band in the visible light field with a peak value of 537 nm, characteristic of pink color. The study dose range of the system is 1 to 110 kGy, however, the linear part up to 10 kGy. Whereas in its liquid state, it has a visible light zone absorption band that rises to 526 nm and is bleached when exposed to γ-rays. Furthermore, The study dose range is 0.3 to 10 kGy (the linear parts up to 2 kGy). Finally, the third dosimetry system is based on the same material as a gel/DTH and a pink hydrogel has a maximum peak of 530 nm, which is fully bleached after irradiation, as well as the study dose range is 50–2200 Gy (the linear parts up to 200 Gy). Depending on the results, a gel dosimeter is additionally more sensitive than both liquid and film dosimeters, due to its low dose range, and sensitivity for low gamma rays.
The G-value was calculated for the three prepared dosimeters and it was found that the values of radiation, chemical yield were higher in the gel/DTHdosimeters and moderately in DTH-liquid dosimeters but low in the DTH film dosimeters showing its high degradation and sensitivity to gamma rays in the form of gel more than liquid and films. The dose range of these three dosimeters relies on the concentration of DTH. All three dosimeters were tested before and after irradiation, and they showed top balance earlier than and after irradiation inside the darkish and light for approximately 63 days for films and liquid and confirmed appropriate balance for gel 30 days. These dosimeters have the advantages of easy to prepare, inexpensive raw materials, as well as the absence of toxic substances. For these characteristics, these techniques can be applied to seed production, fresh food irradiation, radiotherapy applications, medical sterilization, and food irradiation processing.
References
IAEA (1987) Absorbed dose determination in photon and electron beams, Technical Reports Series No. 277, IAEA, Vienna
Gafar SM, Abdel-Kader NM (2020) Dosimetric characteristics and applications of cross-linking and degradation of a natural biopolymer Gum Acacia. Radiochim Act 108(3):223–229
ISO/ASTM 52701 (2020) Standard guide for performance characterization of dosimeters and dosimetry systems for use in radiation processing. 2020. ASTM International, West Conshohocken, PA. ASTM E2701
Gafar SM, Abd El-Kader NM, Mohamed TM (2020) Radiation-induced Bismuth nanoparticles and its possible use as radiation dosimeter. Radiat Effect Defect Soli 175(5–6):529–539
Gafar SM, El-Ahdal MA (2014) Dosimetric characteristics of 2,6 di-nitro phenol for high dose Dosimetry. Dye Pigm 109:67–71
Oberoi PR, Fuke CA, Maurya CB, Mahanwar PA (2020) Comparative study of two azo dyes using Triphenyl-Tetrazolium Chloride (TTC) on gamma irradiation induced film dosimeter. Nucl Instr Meth Phys Res Sec B Beam Interact Mater Atoms 466:82–89
Gafar SM, El-Kelany M, El-Ahdal M (2017) Low-dose film dosimeter based on mixture of AY and TBPE dyed poly(vinyl alcohol). Dyes Pigm 140:1–5
Rabaeh KA, Basfar AA (2020) A polystyrene film dosimeter containing dithizone dye for high dose applications of gamma-ray source. Radiat Phys Chem 170:108646
Rammah YS, Ibrahim SE, Awad EM (2019) Electrical and optical properties of Makrofol DE 1–1 polymeric films induced by gamma irradiation. Bull Natl Res Centre 43(1):212–218
Ali-Omer MA, Ali-Bashir EA (2018) Synthesis of polyvinyl alcohol and cuprous oxide (PVA/Cu2O) films for radiation detection and personal dosimeter based on optical properties. J Radiat Res Appl Sci 11(3):237–241
Raouafi, Daoudi M, Jouini K, Charradi K, Hamzaoui AH, Blaise P (2018) Effect of gamma irradiation on the color, structure and morphology of nickel-doped polyvinyl alcohol films: alternative use as dosimeter or irradiation indicator. Nucl Instrum Methods B 425:4–10
Sobhy A, Faheem E, Gafar SM (2019) Dosimetric studies and chemical kinetics of Resazurin dye and its possible use as radiation dosimeter. Radioanal Nucl Chem 45:1–7
Beshir WB, Eid S, Gafar SM, Ebraheem S (2014) Application of solutions of Rhodamine B in dosimetry. Appl Radiat Isot 89:13–17
D’Arienzo M, Pimpinella M, De Coste V, Capogni M, Ferrari P, Ca Mariotti F, Iaccarino G, Ungania S, Strigari L (2020) Absorbed dose measurements from a 90Y radionuclide liquid solution using LiF:Mg, Cu, P thermoluminescent dosimeters. Physica Med 69(2020):127–133
Gafar SM, El-Kelany MA, El-Shawadfy SR (2018) Spectrophotometric properties of azo dye metal complex and its possible use as radiation dosimeter. J Radiat Res Appl Sci 11(3):190–194
Magdy N, Gafar SM (2022) Development of two dosimetry systems based on basic violet dye for possible use as radiation dosimeters. Pigm Res Techn 51(2):204–211
Krapfenbauer K, Wolfger H, Getoff N, Hamblett I, Navaratnam S (2000) Pulse radiolysis and chemical analysis of azo dyes in aqueous solution I. p-Phenylazoaniline. J Radiat Phys Chem 58:21–27
Rabaeh KA, Aljammal SA, Eyadeh MM, Abumurad KM (2021) Methyl thymol blue solution and film dosimeter for high dose measurements. Radiat Phys Chem 23:123–129
Rabaeha KA, Eyadehb MM, Hailatc TF, Aldwerid FM, Alheete SM, Eid RM (2018) “Characterization of ferrous-methylthymol blue-polyvinyl alcohol gel dosimeters using nuclear magnetic resonance and optical techniques. Radiat Phys Chem 148:25–32
Basfar AA, Moftah B, Rabaeh KA, Almousa AA (2015) Novel composition of polymer gel dosimeters based on N-(Hydroxymethyl) acrylamide for radiation therapy. Radiat Phys Chem 112:117–120
Abtahi SMM, Langaroodi RKS, Akbari ME (2020) Dose distribution verification in intraoperative radiation therapy using an N-isopropyl acrylamide-based polymer gel dosimeter. J Radioanal Nucl Chem, 1–8
Gafar SM, El-Ahdal MA (2015) Radiochromic fuchsine-gel and its possible use for low dosimetry applications. Adv Polym Technol 35(2):146–151
Atia AI, Gafar SM (2020) Radiation_induced free radicals from different milk powders and its possible use as radiation dosimeters milk powder. Radiochim Acta 108(4):321–325
Karakirova Y, Yordanova V (2021) Optimizing the size of cylindrical sucrose solid state/EPR dosimeters for ionizing radiation. Radiat Phys Chem 184:1–6
Çemberci M, Bıyık R, Fidan M, Tapramaz R (2012) EPR Study of UV and gamma irradiated potassium persulfate: a Sensitive dosimeter. Radiat Meas 146(2021):106–111
Taguchi M, Kojima T (2007) Yield of OH radicals in water under heavy ion irradiation. Dependence on mass, specific energy, and elapsed time. J Nucl Sci Tech 18:35–38
Zaki A (2006) Usability of aqueous solutions of methyl red as high-dose dosimeter for gamma radiation. Radiat Meas 41:438–442
El-halawany N, Wassel AR, Abdelhamid, AE, Elfadl AA, Nouh S (2020) Novel hyper branched polyanilinenanocomposites for gamma radiation dosimetry, 31: 5914–5925
ASTM E1026 (2004) Practice for using the Fricke reference standard dosimetry system. Annual Book of ASTM Standards. ASTM International, West Conshohocken, PA
McLaughlin WL, Desrosiers MF (1995) Dosimetry systems for radiation processing. Radiat Phys Chem 46(4–6):163–174
Acknowledgements
This work was financially supported by the National Center for Radiation Research and Technology (NCRRT), the Egyptian Atomic Energy Authority (EAEA), Nasr City, Cairo, Egypt for basic research in the field of radiation technology applications.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest. We alone are responsible for the content and writing of the paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gafar, S.M., El-Ahdal, M.A. & El-Shawadfy, S.R. Variation in dose response of three dosimetry systems based on diphenyl thiocarbazone. J Radioanal Nucl Chem 331, 3391–3399 (2022). https://doi.org/10.1007/s10967-022-08392-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-022-08392-0