Abstract
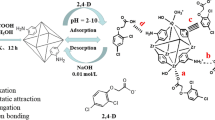
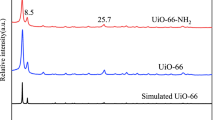
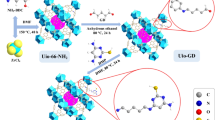
A zirconium-based metal–organic framework (DUT-69) was fabricated via a hydrothermal synthesis for U(VI) removal in aqueous solutions. Experimental results showed that the maximum adsorption capacity for U(VI) was 362.32 mg·g−1 at 303 K, pH = 6 and initial U(VI) concentration of 80 mg L−1. The adsorption process fit well with the pseudo-second-order kinetic and Langmuir models. Various characterizations indicated that complexation interactions was the central adsorption mechanism and electrostatic was the secondary. The carboxyl, Zr–O, and C–S bonds in the framework participated in the adsorption process. Reusability experiments showed that 80.34% adsorption rate could be maintained after 5 cycles.









Similar content being viewed by others
References
Tang N, Liang J, Niu C, Wang H, Luo Y, Xing W, Ye S, Liang C, Guo H, Guo J, Zhang Y, Zeng G (2020) Amidoxime-based materials for uranium recovery and removal. J Mater Chem A 8(16):7588–7625. https://doi.org/10.1039/c9ta14082d
Xie Y, Chen C, Ren X, Wang X, Wang H, Wang X (2019) Emerging natural and tailored materials for uranium-contaminated water treatment and environmental remediation. Prog Mater Sci 103:180–234. https://doi.org/10.1016/j.pmatsci.2019.01.005
Li FF, Cui WR, Jiang W, Zhang CR, Liang RP, Qiu JD (2020) Stable sp(2) carbon-conjugated covalent organic framework for detection and efficient adsorption of uranium from radioactive wastewater. J Hazard Mater 392:122333. https://doi.org/10.1016/j.jhazmat.2020.122333
Dariusz S, Agnieszka G-P, Ewelina G, Marek M, Waldemar K (2019) Study of effect of phosphate and uranium ions on the thermal properties of surfactant-modified natural red clay using TG–FTIR–MS techniques. J Therm Anal Calorim 136:425–439. https://doi.org/10.1007/s10973-018-7616-x
Nekhunguni PM, Tavengwa NT, Tutu H (2017) Sorption of uranium(VI) onto hydrous ferric oxide-modified zeolite: Assessment of the effect of pH, contact time, temperature, selected cations and anions on sorbent interactions. J Environ Manage 204(Pt 1):571–582. https://doi.org/10.1016/j.jenvman.2017.09.034
Yu B, Ye G, Chen J, Ma S (2019) Membrane-supported 1D MOF hollow superstructure array prepared by polydopamine-regulated contra-diffusion synthesis for uranium entrapment. Environ Pollut 253:39–48. https://doi.org/10.1016/j.envpol.2019.06.114
Li J, Wang X, Zhao G, Chen C, Chai Z, Ahmed A, Tasawar H, Wang X (2018) Metal-organic framework-based materials: superior adsorbents for the capture of toxic and radioactive metal ions. Chem Soc Rev 47(7):2322–2356. https://doi.org/10.1039/c7cs00543a
Camille P (2018) Present and future of MOF research in the field of adsorption and molecular separation. Curr Opin Chem Eng 20:132–142. https://doi.org/10.1016/j.coche.2018.04.004
Qi W, Qiaoyuan G, Al-Enizi AM, Ayman N, Shengqian M (2020) Recent advances in MOF-based photocatalysis: environmental remediation under visible light. Inorg Chem Front 7(2):300–339. https://doi.org/10.1039/c9qi01120j
Kun R, Guoqiang Z, Yaping C, Yue L, Qian Z, Jiayi C, Jixin Z, Wenping S, Wei H, ShiXue D (2018) Hybrid 2D dual-metal–organic frameworks for enhanced water oxidation catalysis. Adv Func Mater 28(26):1801554. https://doi.org/10.1002/adfm.201801554
Wuttke S, Lismont M, Escudero A, Rungtaweevoranit B, Parak WJ (2017) Positioning metal-organic framework nanoparticles within the context of drug delivery—A comparison with mesoporous silica nanoparticles and dendrimers. Biomaterials 123:172–183. https://doi.org/10.1016/j.biomaterials.2017.01.025
De Decker J, Rochette J, De Clercq J, Florek J, Van Der Voort P (2017) Carbamoylmethylphosphine oxide-functionalized MIL-101(cr) as highly selective uranium adsorbent. Anal Chem 89(11):5678–5682. https://doi.org/10.1021/acs.analchem.7b00821
Xiaomei Z, Ying L, Yan J, Qianhong G, Peng W, Yi Y (2019) Enhanced selectively removal uranyl ions from aqueous solution by Fe@ZIF-8. Microporous Mesoporous Mater 277:52–59. https://doi.org/10.1016/j.micromeso.2018.10.017
Wang Z, Zhao D, Wu C, Chen S, Wang Y, Chen C (2020) Magnetic metal organic frameworks/graphene oxide adsorbent for the removal of U(VI) from aqueous solution. Appl Radiat Isot 162:109160. https://doi.org/10.1016/j.apradiso.2020.109160
Rao Z, Feng K, Tang B, Wu P (2017) Surface decoration of amino-functionalized metal-organic framework/graphene oxide composite onto polydopamine-coated membrane substrate for highly efficient heavy metal removal. ACS Appl Mater Interfaces 9(3):2594–2605. https://doi.org/10.1021/acsami.6b15873
Zhao X, Zhao Y, Zheng M, Liu S, Xue W, Du G, Wang T, Gao X, Wang K, Hu J, Gao Z, Huang H (2019) Efficient separation of vitamins mixture in aqueous solution using a stable zirconium-based metal-organic framework. J Colloid Interface Sci 555:714–721. https://doi.org/10.1016/j.jcis.2019.08.024
Cavka JH, Jakobsen S, Olsbye U, Guillou N, Lamberti C, Bordiga S, Lillerud KP (2008) A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J Am Chem Soc 130(42):13850–13851. https://doi.org/10.1021/ja8057953
Geisse AR, Ngule CM, Genna DT (2020) Removal of lead ions from water using thiophene-functionalized metal–organic frameworks. Chem Commun 56(2):237–240. https://doi.org/10.1039/c9cc09022c
He T, Zhang YZ, Kong XJ, Yu J, Lv XL, Wu Y, Guo ZJ, Li JR (2018) Zr(IV)-based metal-organic framework with T-shaped ligand: unique structure, high stability, selective detection, and rapid adsorption of Cr2O72- in water. ACS Appl Mater Interfaces 10(19):16650–16659. https://doi.org/10.1021/acsami.8b03987
Mondloch JE, Katz MJ, Planas N, Semrouni D, Gagliardi L, Hupp JT, Farha OK (2014) Are Zr(6)-based MOFs water stable? Linker hydrolysis versus capillary-force-driven channel collapse. Chem Commun (Camb) 50(64):8944–8946. https://doi.org/10.1039/c4cc02401j
Yuan S, Feng L, Wang K, Pang J, Bosch M, Lollar C, Sun Y, Qin J, Yang X, Zhang P, Wang Q, Zou L, Zhang Y, Zhang L, Fang Y, Li J, Zhou HC (2018) Stable metal–organic frameworks: design, synthesis, and applications. Adv Mater 30(37):e1704303. https://doi.org/10.1002/adma.201704303
Bon V, Senkovska I, Baburin IA, Kaskel S (2013) Zr- and Hf-based metal–organic frameworks: Tracking down the polymorphism. Cryst Growth Des 13(3):1231–1237. https://doi.org/10.1021/cg301691d
Tao W, Aiyun H, Guangzhi X, Chen L, Haijun W, Yongmei X (2019) Porous Zr–thiophenedicarboxylate hybrid for catalytic transfer hydrogenation of bio-based furfural to furfuryl alcohol. Catal Lett 149(7):1845–1855. https://doi.org/10.1007/s10562-019-02748-0
Drache F, Bon V, Senkovska I, Getzschmann J, Kaskel S (2017) The modulator driven polymorphism of Zr(IV) based metal-organic frameworks. Philos Trans R Sci A 375(2084):20160027. https://doi.org/10.1098/rsta.2016.0027
Xiaoyang L, Shuibo X, Guohua W, Xin H, Yi D, Haiyan L (2020) Fabrication of environmentally sensitive amidoxime hydrogel for extraction of uranium (VI) from an aqueous solution. Colloids Surf A Physicochem Eng Asp. https://doi.org/10.1016/j.colsurfa.2020.125813
Jian JM, Zhang C, Wang F, Lu X, Wang F, Zeng EY (2019) Effect of solution chemistry and aggregation on adsorption of perfluorooctanesulphonate (PFOS) to nano-sized alumina. Environ Pollut 251:425–433. https://doi.org/10.1016/j.envpol.2019.05.025
Li H, Liu X, Yang T, Zhao W, Saravanamurugan S, Yang S (2017) Porous zirconium-furandicarboxylate microspheres for efficient redox conversion of biofuranics. Chemsuschem 10(8):1761–1770. https://doi.org/10.1002/cssc.201601898
Drache F, Cirujano FG, Nguyen KD, Bon V, Senkovska I, Llabrés i Xamena FX, Kaskel S (2018) Anion exchange and catalytic functionalization of the zirconium-based metal–organic framework DUT-67. Cryst Growth Des 18(9):5492–5500. https://doi.org/10.1021/acs.cgd.8b00832
Wei J, Zhang W, Pan W, Li C, Sun W (2018) Experimental and theoretical investigations on Se(iv) and Se(vi) adsorption to UiO-66-based metal–organic frameworks. Environ Sci Nano 5(6):1441–1453. https://doi.org/10.1039/c8en00180d
Yin LL, Kong XY, Zhang Y, Ji YQ (2018) Facile synthesis of the magnetic metal organic framework Fe3O4@UiO-66-NH2 for separation of strontium. Biomed Environ Sci 31(6):483–488. https://doi.org/10.3967/bes2018.065
Li R, Che R, Liu Q, Su S, Li Z, Zhang H, Liu J, Liu L, Wang J (2017) Hierarchically structured layered-double-hydroxides derived by ZIF-67 for uranium recovery from simulated seawater. J Hazard Mater 338:167–176. https://doi.org/10.1016/j.jhazmat.2017.04.075
Liu Y, Zou J, Guo B, Ren Y, Wang Z, Song Y, Yu Y, Wu L (2020) Selective Photocatalytic Oxidation of Thioanisole on DUT-67(Zr) Mediated by Surface Coordination. Langmuir 36(9):2199–2208. https://doi.org/10.1021/acs.langmuir.9b02582
Zhu W, Li Y, Dai L, Li J, Li X, Li W, Duan T, Lei J, Chen T (2018) Bioassembly of fungal hyphae/carbon nanotubes composite as a versatile adsorbent for water pollution control. Chem Eng J 339:214–222. https://doi.org/10.1016/j.cej.2018.01.134
Lee YR, Yu K, Ravi S, Ahn WS (2018) Selective adsorption of rare earth elements over functionalized Cr-MIL-101. ACS Appl Mater Interfaces 10(28):23918–23927. https://doi.org/10.1021/acsami.8b07130
Li M, Liu H, Chen T, Dong C, Sun Y (2019) Synthesis of magnetic biochar composites for enhanced uranium(VI) adsorption. Sci Total Environ 651:1020–1028. https://doi.org/10.1016/j.scitotenv.2018.09.259
Wang X, Yu S, Wu Y, Pang H, Yu S, Chen Z, Hou J, Alsaedi A, Hayat T, Wang S (2018) The synergistic elimination of uranium (VI) species from aqueous solution using bi-functional nanocomposite of carbon sphere and layered double hydroxide. Chem Eng J 342:321–330. https://doi.org/10.1016/j.cej.2018.02.102
Li L, Ma W, Shen S, Huang H, Bai Y, Liu H (2016) A Combined experimental and theoretical study on the extraction of uranium by amino-derived metal–organic frameworks through post-synthetic strategy. ACS Appl Mater Interfaces 8(45):31032–31041. https://doi.org/10.1021/acsami.6b11332
Feng ML, Sarma D, Qi XH, Du KZ, Huang XY, Kanatzidis MG (2016) Efficient removal and recovery of uranium by a layered organic-inorganic hybrid thiostannate. J Am Chem Soc 138(38):12578–12585. https://doi.org/10.1021/jacs.6b07351
Lin Z-J, Zheng H-Q, Zeng Y-N, Wang Y-L, Chen J, Cao G-J, Gu J-F, Chen B (2019) Effective and selective adsorption of organoarsenic acids from water over a Zr-based metal-organic framework. Chem Eng J 378:122196. https://doi.org/10.1016/j.cej.2019.122196
Liao Y, Wang M, Chen D (2018) Production of three-dimensional porous polydopamine-functionalized attapulgite/chitosan aerogel for uranium(VI) adsorption. J Radioanal Nucl Chem 316(2):635–647. https://doi.org/10.1007/s10967-018-5816-2
Alqadami AA, Naushad M, Alothman ZA, Ghfar AA (2017) Novel metal–organic framework (MOF) based composite material for the sequestration of U(VI) and Th(IV) metal ions from aqueous environment. ACS Appl Mater Interfaces 9(41):36026–36037. https://doi.org/10.1021/acsami.7b10768
Ding L, Shao P, Luo Y, Yin X, Yu S, Fang L, Yang L, Yang J, Luo X (2020) Functionalization of UiO-66-NH2 with rhodanine via amidation: Towarding a robust adsorbent with dual coordination sites for selective capture of Ag(I) from wastewater. Chem Eng J. https://doi.org/10.1016/j.cej.2019.123009
Yin L, Wang P, Wen T, Yu S, Wang X, Hayat T, Alsaedi A, Wang X (2017) Synthesis of layered titanate nanowires at low temperature and their application in efficient removal of U(VI). Environ Pollut 226:125–134. https://doi.org/10.1016/j.envpol.2017.03.078
Huang S, Pang H, Li L, Jiang S, Wen T, Zhuang L, Hu B, Wang X (2018) Unexpected ultrafast and high adsorption of U(VI) and Eu(III) from solution using porous Al2O3 microspheres derived from MIL-53. Chem Eng J 353:157–166. https://doi.org/10.1016/j.cej.2018.07.129
De Decker J, Folens K, De Clercq J, Meledina M, Van Tendeloo G, Du Laing G, Van Der Voort P (2017) Ship-in-a-bottle CMPO in MIL-101(Cr) for selective uranium recovery from aqueous streams through adsorption. J Hazard Mater 335:1–9. https://doi.org/10.1016/j.jhazmat.2017.04.029
Wu H, Chi F, Zhang S, Wen J, Xiong J, Hu S (2019) Control of pore chemistry in metal-organic frameworks for selective uranium extraction from seawater. Microporous Mesoporous Mater 288:109567. https://doi.org/10.1016/j.micromeso.2019.109567
Zhang J-Y, Zhang N, Zhang L, Fang Y, Deng W, Yu M, Wang Z, Li L, Liu X, Li J (2015) Adsorption of uranyl ions on amine-functionalization of mil-101(cr) nanoparticles by a facile coordination-based post-synthetic strategy and x-ray absorption spectroscopy studies. Sci Rep 5:13514. https://doi.org/10.1038/srep13514
Luo B-C, Yuan L-Y, Chai Z-F, Shi W-Q, Tang Q (2015) U(VI) capture from aqueous solution by highly porous and stable MOFs: UiO-66 and its amine derivative. J Radioanal Nucl Chem 307(1):269–276. https://doi.org/10.1007/s10967-015-4108-3
Yang P, Liu Q, Liu J, Zhang H, Li Z, Li R, Liu L, Wang J (2017) Interfacial growth of a metal–organic framework (UiO-66) on functionalized graphene oxide (GO) as a suitable seawater adsorbent for extraction of uranium(vi). J Mater Chem A 5(34):17933–17942. https://doi.org/10.1039/c6ta10022h
Fotovat H, Khajeh M, Oveisi AR, Ghaffari-Moghaddam M, Daliran S (2018) A hybrid material composed of an amino-functionalized zirconium-based metal-organic framework and a urea-based porous organic polymer as an efficient sorbent for extraction of uranium(VI). Mikrochim Acta 185(10):469. https://doi.org/10.1007/s00604-018-2991-3
Zhao F, Repo E, Song Y, Yin D, Hammouda SB, Chen L, Kalliola S, Tang J, Tam KC, Sillanpää M (2017) Polyethylenimine-cross-linked cellulose nanocrystals for highly efficient recovery of rare earth elements from water and a mechanism study. Green Chem 19(20):4816–4828. https://doi.org/10.1039/c7gc01770g
Shuo L, Yufeng Z, Kwame BJ, Chul-Woong C, Kumar SA, Che-Ryong L, Yeoung-Sang Y (2019) Structure-controlled recovery of palladium(II) from acidic aqueous solution using metal-organic frameworks of MOF-802, UiO-66 and MOF-808. Chem Eng J 362:280–286. https://doi.org/10.1016/j.cej.2019.01.044
Acknowledgments
The authors are grateful to the Laboratory of Pollution Control and Resource Technology and the Department of Municipal Engineering at the University of South China for providing the necessary facilities for the research.
Funding
This research was supported by Youth Program of National Natural Science Foundation of China (NO.51904155) and the key scientific research project of Education Bureau of Hunan Province (19A421).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, X., Xie, S., Wang, G. et al. The performance and mechanism of U(VI) removal from aqueous solutions by a metal–organic framework (DUT-69). J Radioanal Nucl Chem 328, 181–194 (2021). https://doi.org/10.1007/s10967-021-07645-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-021-07645-8