Abstract
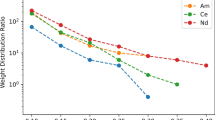
The extraction of radium and actinium with Eichrom Pb resin and Rose Bengal has been studied. Radium and actinium both show a strong affinity (k’ > 10,000) for the resin at intermediate pHs (~ 5–7) from solutions with Rose Bengal. The pH range and magnitude of uptake for both radium and actinium is significantly increased by the presence of Rose Bengal compared to basic solutions without this counter ion. Radium uptake is extremely fast from basic solutions with and without Rose Bengal; actinium uptake is slower, particularly in solutions without Rose Bengal. Column studies show that despite the similar behavior of actinium and radium in this system, separations of these two elements with Pb resin and Rose Bengal solutions are possible with reasonable yields due to the differences in uptake kinetics.









Similar content being viewed by others
References
Filosofov D, Lebedev N, Radchenko V, Rakhimov A, Happel S, Roesch F (2015) Behavior of actinium, alkaline and rare earth elements in Sr- resin/mineral acid systems. Solvent Extr Ion Exch 00:1–14
Mohamud H, van Es E, Sainsbury T, Ivanov P, Russell B, Regan P, Ward N (2017) Progress towards the development of a rapid analytical approach for separation of 226Ra using dibenzo-18-crown-6 ether functionalised silica (SiO2) disks. Radiat Phys Chem 140:57–60
Thiele N, Wilson J (2018) Actinum-225 for targeted alpha therapy: coordination chemistry and current chelation approaches. Cancer Biother Radiopharm 33(8):336–347
Gott M, Steinbach J, Mamat C (2016) The radiochemical and radiopharmaceutical applications of radium. Open Chem 14(1):118–129
Despotopulos J, Kmak K, Gharibyan N, Henderson R, Moody K, Shaughnessy D, Sudowe R (2016) Characterization of the homologs of flerovium with crown ether based extraction chromatography resins: studies in hydrochloric acid. J Radioanal Nucl Chem 310:1201–1207
Imura H, Saito Y, Ohashi K, Meguro Y, Yoshida Z, Choppin G (1996) Characterization of the Lanthanum(III) and Europium(III) tricholoacetate complexes extracted with 18-crown-6. Solvent Extr Ion Exch 14(5):817–832
Parham H, Fazeli A (2000) Extraction-spectrophotometric determination of trace amounts of barium by 18-crown-6 and Rose Bengal. Anal Sci 16:575–577
Bilski P, Chignell C (1994) Properties of differently charged micelles containing rose bengal: application in photosensitization studies. J Photochem Photobiol A: Chem 77:49–58
Lambert C, Kochevar I (1997) Electron transfer quenching of the rose bengal triplet state. Photochem Photobiol 66(1):15–25
Schoolaert E, Steyaert I, Vancoillie G, Geltmeyer J, Lava K, Hoogenboom R, De Clerck K (2016) Blend electrospinning of dye-functionalized chitosan and poly(e-caprolactone): towards biocompatible pH-sensors. J Mater Chem B 4:4507
Horwitz E, Dietz M, Rhoads S, Felinto C, Gale N, Houghton J (1994) A lead-selective extraction chromatographic resin and its application to the isolation of lead from geological samples. Anal Chim Acta 292:263–273
National Nuclear Data Center “NNDC” (2019) Brookhaven National Laboratory. https://www.nndc.bnl.gov/nudat2/indx_dec.jsp. Accessed 6 November 2020
Horwitz E, Bloomquist C (1972) The preparation, performance and factors affecting band spreading of high efficiency extraction chromatographic columns for actinide separations. J Inorg Nucl Chem 34:3851–3871
Lamberts J, Schumacher D, Neckers D (1984) Novel rose bengal derivatives: synthesis and quantum yield studies. J Am Chem Soc 106:5879–5883
Chen X, Ji M, Fisher D, Wai C (1999) Ionizable calixarene-crown ethers with high selectivity for radium over light alkaline earth metal ions. Inorg Chem 38(23):5449–5452
Shannon R (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr A A32:751–767
Takeda Y, Kawarabayashi A, Endo K, Yahata T, Kudo Y, Katsuta S (1998) Solvent extraction of alkali metal (Li-Cs) picrates with 18-crown-6 into various diluents. Elucidation of fundamental equilibria which govern the extraction-ability and-selectivity. Anal Sci 14:215–223
Hancock R, Siddons C, Oscarson K, Reibenspies J (2004) The structure of the 11-coordinate barium complex of the pendant-donor macrocycle 1,4,7,10-tetrakis(carbamoylmethyl)-1,4,7,10-tetraazacyclododecane: an analysis of the coordination numbers of barium(II) in its complexes. Inorg Chim Acta 357(3):723–727
Morgenstern A, Lilley L, Stein B, Kozimor S, Batista E, Yang P (2020) Computer-assisted design of macrocyclic chelators for actinium-225 radiotherapeutics. Inorganic Chem (Online). https://doi.org/10.1021/acs.inorgchem.0c02432
Zielinska B, Bilewicz A (2004) The hydrolysis of actinium. J Radioanly Nucl Chem 261(1):195–198
Kirby H, Salutsky M (1964) The Radiochemistry of Radium. The National Academies Press, Washington, DC
Acknowledgements
This study was performed under the auspices of the U.S. Department of Energy by Lawrence Livermore National Laboratory under Contract DE-AC52-07NA27344. This work was funded by the LLNL Livermore Graduate Research Scholar Program. This material is based upon work supported by the Department of Energy National Nuclear Security Administration through the Nuclear Science and Security Consortium under Award Number DE-NA0003180. The authors would like to thank Roger Henderson for providing the nescessary isotopes and Carlos Valdez for providing the Rose Bengal.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kmak, K.N., Shaughnessy, D.A. & Vujic, J. Extraction of radium and actinium with Pb resin and Rose Bengal. J Radioanal Nucl Chem 328, 377–385 (2021). https://doi.org/10.1007/s10967-021-07642-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-021-07642-x