Abstract
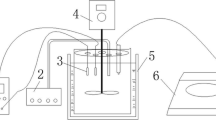
In this study prussian blue (PB)/ferrite composites by in situ electrosynthesis were used to remove cesium (Cs) from aqueous radioactive waste. The effect of various reaction conditions on Cs+ removal effect was investigated by measuring residual Cs concentration using ICP-MS, exploring the reaction mechanism by using SEM–EDS, TEM, FT-IR, XRD and VSM. The results show it was feasible to remove Cs+ from simulated radioactive aqueous waste by in situ electrosynthesis of magnetic ferrocyanide composites. The best removal efficiency was 99.70%. The composites consist of a mixture of PB, CsFe[Fe(CN)6] and Fe3O4. Meanwhile, composites can rapidly separated from aqueous waste by magnets.








Similar content being viewed by others
References
Yang H, Du L, Tian X, Fan Z, Sun C, Liu Y, Keelan JA, Nie G (2014) Effects of nanoparticle size and gestational age on maternal biodistribution and toxicity of gold nanoparticles in pregnant mice. Toxicol Lett 230(1):10–18
Yang H, Lei S, Zhai J, Li H, Yu H (2013) In situ controllable synthesis of magnetic prussian blue/graphene oxide nanocomposites for removal of radioactive cesium in water. J Mater Chem A 2(2):326–332
Assimakopoulos PA, Ioannides KG, Karamanis DT, Pakou AA, Stamoulis KC, Vayonakis A, Veltsos E (1993) Time dependence of the transfer factor of 137Cs from surface soil to plants. Sci Total Environ 138(1–3):309–315
Andersson KG, Roed J (1994) The behaviour of chernobyl 137 Cs, 134 Cs and 106 Ru in undisturbed soil: implications for external radiation. J Environ Radioact 22(3):183–196
Daburon F, Archimbaud Y, Cousi J, Fayart G, Hoffschir D, Chevallereau I, Creff HL, Gueguen L (1991) Radiocaesium transfer to ewes fed contaminated hay after the chernobyl accident: effect of vermiculite and AFCF (ammonium ferricyanoferrate) as countermeasures. J Environ Radioact 14(1):73–84
Ratnikov AN, Vasiliev AV, Alexakhin RM, Krasnova EG, Pasternak AD, Howard BJ, Hove K, Strand P (1998) The use of hexacyanoferrates in different forms to reduce radiocaesium contamination of animal products in Russia. Sci Total Environ 223(2–3):167–176
Thammawong C, Opaprakasit P, Tangboriboonrat P, Sreearunothai P (2013) Prussian blue-coated magnetic nanoparticles for removal of cesium from contaminated environment. J Nanopart Res 15(6):1689
Jeon C (2016) Removal of cesium ions from aqueous solutions using immobilized nickel hexacyanoferrate-sericite beads in the batch and continuous processes. J Ind Eng 40:93–98
Zou Y, Wang X, Khan A, Wang P, Liu Y, Alsaedi A, Hayat T, Wang X (2016) Environmental remediation and application of nanoscale zero-valent iron and its composites for the removal of heavy metal ions: a review. Environ Sci Technol 50(14):7290–7304
Li J, Wang X, Zhao G, Chen C, Wang X (2018) Metal–organic framework-based materials: superior adsorbents for the capture of toxic and radioactive metal ions. Chem Soc Rev 47(7):2322–2356
Liu X, Chen GR, Lee DJ, Kawamoto T, Tanaka H, Chen ML, Luo YK (2014) Adsorption removal of cesium from drinking waters: a mini review on use of biosorbents and other adsorbents. Bioresour Technol 160(5):142–149
Nogami M, Sugiyama Y, Kawasaki T, Harada M, Morita Y, Kikuchi T, Ikeda Y (2010) Adsorptivity of polyvinylpolypyrrolidone for selective separation of U(VI) from nitric acid media. J Radioanal Nucl Chem 283(2):541–546
Özeroğlu C, Bilgiç ÖD (2015) Use of the crosslinked copolymer functionalized with acrylic acid for the removal of strontium ions from aqueous solutions. J Radioanal Nucl Chem 305(2):551–565
Venkatesan KA, Shymala KV, Antony MP, Srinivasan TG, Rao PRV (2008) Batch and dynamic extraction of uranium(VI) from nitric acid medium by commercial phosphinic acid resin, Tulsion Ch-96. J Radioanal Nucl Chem 275(3):563–570
Özeroğlu C, Keçeli G (2009) Kinetic and thermodynamic studies on the adsorption of U(VI) ions on densely crosslinked poly(methacrylic acid) from aqueous solutions. Radiochim Acta 97:709–717
Karadağ E, Saraydın D, Güven O (1995) Behaviors of acrylamide/itaconic acid hydrogels in uptake of uranyl ions from aqueous solutions. Sep Sci Technol 30(20):3747–3760
Özeroğlu C, Doğan E, Keçeli G (2011) Investigation of Cs(I) adsorption on densely crosslinked poly(sodium methacrylate) from aqueous solutions. J Radioanal Nucl Chem 289(2):577–586
Metilda P, Sanghamitra K, Gladis JM, Naidu GRK, Rao TP (2005) Amberlite XAD-4 functionalized with succinic acid for the solid phase extractive preconcentration and separation of uranium(VI). Talanta 66:192–200
Kazemian H, Zakeri H (2006) Rabbani MS Cs and Sr removal from solution using potassium nickel hexacyanoferrate impregnated zeolites. J Radioanal Nucl Chem 268(2):231–236
Torad NL, Hu M, Imura M, Naito M, Yamauchi Y (2012) Large Cs adsorption capability of nanostructured prussian blue particles with high accessible surface areas. J Radioanal Nucl Chem 22(35):18261–18267
Li B, Liao B, Wu JL, Zhang JJ, Zhao D, Jun ZH (2008) Removal of radioactive cesium from solutions by zinc ferrocyanide. Sci Technol 19(2):88–92
Loos-Neskovic C, Ayrault S, Badillo V, Jimenez B, Garnier E, Fedoroff M, Jones DJ, Merinov B (2004) Structure of copper–potassium hexacyanoferrate (II) and sorption mechanisms of cesium. J Solid State Chem 177(6):1817–1828
Sasaki T, Tanaka S (2011) Magnetic separation of cesium ion using prussian blue modified magnetite. Chem Lett 41(1):32–34
Chang L, Chang S, Chen W, Han W, Li Z, Zhang Z, Dai Y, Chen D (2016) Facile one-pot synthesis of magnetic prussian blue core/shell nanoparticles for radioactive cesium removal. RSC Adv 6(98):96223–96228
Huang G, Dou P, Zhen Z, Jing Y (2018) Removal of cobalt from liquid radioactive waste by in situ electrochemical synthesis of ferrite. J Radioanal Nucl Chem 316(1):61–70
Barale M, Lefèvre G, Carrette F, Catalette H, Fédoroff M, Cote G (2008) Effect of the adsorption of lithium and borate species on the zeta potential of particles of cobalt ferrite, nickel ferrite, and magnetite. J Comb Chem 328(1):34–40
Cabrera L, Gutierrez S, Menendez N, Morales MP, Herrasti P (2008) Magnetite nanoparticles: electrochemical synthesis and characterization. Electrochim Acta 53(8):3436–3441
Parajuli D, Takahashi A, Noguchi H, Kitajima A, Tanaka H, Takasaki M, Yoshino K, Kawamoto T (2016) Comparative study of the factors associated with the application of metal hexacyanoferrates for environmental Cs decontamination. Chem Eng J 283:1322–1328
Yang Y, Faustino PJ, Progar JJ, Brownell CR, Sadrieh N, May JC, Leutzinger E, Place DA, Duffy EP, Lawrence XY (2008) Quantitative determination of thallium binding to ferric hexacyanoferrate: prussian blue. Int J Pharm 353(1–2):187–194
Wang J, Deng T, Dai Y (2005) Study on the processes and mechanism of the formation of Fe3O4 at low temperature. J Alloys Compd 390(1–2):127–132
Mazario E, Morales M, Galindo R, Herrasti P, Menendez N (2012) Influence of the temperature in the electrochemical synthesis of cobalt ferrites nanoparticles. J Alloys Compd 536:S222–S225
Jia Z, Sun G (2007) Preparation of prussian blue nanoparticles with single precursor. Colloids Surf A 302(1–3):326–329
Jang J, Lee DS (2016) Magnetic prussian blue nanocomposites for effective cesium removal from aqueous solution. Ind Eng Chem Res 55(13):3852–3860
Su JY, Jin GP, Chen T, Liu XD, Chen CN, Tian JJ (2017) The characterization and application of prussian blue at graphene coated carbon fibers in a separated adsorption and electrically switched ion exchange desorption processes of cesium. Electrochim Acta 230:399–406
Wilde RE, Ghosh SN, Marshall BJ (1970) Prussian blues. Inorg Chem 9(11):2512–2516
Hu M, Furukawa S, Ohtani R, Sukegawa H, Nemoto Y, Reboul J, Kitagawa S, Yamauchi Y (2012) Synthesis of prussian blue nanoparticles with a hollow interior by controlled chemical etching. Angew Chem Int Ed 51(4):984–988
Pogorilyi R, Melnyk I, Zub Y, Carlson S, Daniel G, Svedlindh P, Seisenbaeva G, Kessler V (2014) New product from old reaction: uniform magnetite nanoparticles from iron-mediated synthesis of alkali iodides and their protection from leaching in acidic media. Rsc Adv 4(43):22606–22612
Ishizaki M, Akiba S, Ohtani A, Hoshi Y, Ono K, Matsuba M, Togashi T, Kananizuka K, Sakamoto M, Takahashi A (2013) Proton-exchange mechanism of specific Cs+ adsorption via lattice defect sites of prussian blue filled with coordination and crystallization water molecules. Dalton Trans 42(45):16049–16055
Acknowledgements
The authors would like to thank for the help from the analysts at Center of Analysis and Test, Laboratory for Resource and Environmental Education, and School of Chemical Engineering in East China University of Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Huang, G., Chen, J., Dou, P. et al. In situ electrosynthesis of magnetic Prussian blue/ferrite composites for removal of cesium in aqueous radioactive waste. J Radioanal Nucl Chem 323, 557–565 (2020). https://doi.org/10.1007/s10967-019-06966-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-019-06966-z