Abstract
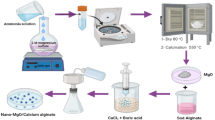
In this study, the core–shell nanoscale zero-valent iron (nZVI)@Alg–Ca beads were synthesized by coaxial electronic injection method for removal of U (VI) from aqueous solution, and characterized by SEM, EDX and XPS. The results showed that the pseudo-second-order models and the Langmuir isotherm model fitted well with the data obtained. The removal mechanism may include both physical adsorption of U (VI) on the surface or inside of core–shell nZVI@Alg–Ca beads and subsequent reduction of U (VI) to U (IV). Therefore, the core–shell nZVI@Alg–Ca beads would have an application prospect in effective removal of U (VI) contamination from aqueous solution.














Similar content being viewed by others
References
Ding S, Yang Y, Li C, Huang H, Hou LA (2016) The effects of organic fouling on the removal of radionuclides by reverse osmosis membranes. Water Res 95:174–184
Francis AJ, Dodge CJ (2008) Bioreduction of uranium (VI) complexed with citric acid by Clostridia affects its structure and solubility. Environ Sci Technol 42:8277–8282
Xie S, Yang J, Chen C, Zhang X, Zhang C (2008) Study on biosorption kinetics and thermodynamics of uranium by Citrobacter freudii. J Environ Radioact 99:126–133
O’Loughlin EJ, Kelly SD, Cook RE, Csencsits R, Kemner KM (2003) Reduction of uranium (VI) by mixed iron (II)/iron (III) hydroxide (green rust): formation of UO2 nanoparticles. Environ Sci Technol 37:721–727
Sheng L, Fein JB (2014) Uranium reduction by Shewanella oneidensis MR-1 as a function of NaHCO3 concentration: surface complexation control of reduction kinetics. Environ Sci Technol 48:3768–3775
O’Loughlin EJ, Kelly SD, Kemner KM (2010) XAFS investigation of the interactions of U(VI) with secondary mineralization products from the bioreduction of Fe(III) oxides. Environ Sci Technol 44:1656–1661
Chen Z, Zhuang Z, Cao Q, Pan X, Guan X, Lin Z (2014) Adsorption-induced crystallization of U-rich nanocrystals on nano-Mg(OH)2 and the aqueous uranyl enrichment. ACS Appl Mater Inter 6:1301–1305
Shi J, Ai Z, Zhang L (2014) Fe@Fe2O3 core-shell nanowires enhanced Fenton oxidation by accelerating the Fe(III)/Fe(II) cycles. Water Res 59:145–153
Nam S, Tratnyek PG (2000) Reduction of azo dyes with zero-valent iron. Water Res 34:1837–1845
He F, Zhao D, Liu J, Roberts CB (2007) Stabilization of Fe-Pd nanoparticles with sodium carboxymethyl cellulose for enhanced transport and dechlorination of trichloroethylene in soil and groundwater. Ind Eng Chem Res 46:29–34
Bezbaruah AN, Krajangpan S, Chisholm BJ, Khan E, Bermudez JJE (2009) Entrapment of iron nanoparticles in calcium alginate beads for groundwater remediation applications. J Hazard Mater 166:1339–1343
Geng B, Jin ZH, Li TL, Qi XH (2009) Kinetics of hexavalent chromium removal from water by chitosan-Fe0 nanoparticles. Chemosphere 75:825–830
Chang C, Lian F, Zhu LY (2011) Simultaneous adsorption and degradation of γ-HCH by nZVI/Cu bimetallic nanoparticles with activated carbon support. Environ Pollut 159:2507–2514
Yu J, Wang JL, Jiang YZ (2016) Removal of Uranium from aqueous solution by alginate beads. Nucl Eng Technol 49(3):534–540
Kuang Y, Du JH, Zhou RB, Chen ZL, Megharaj M, Naidu R (2015) Calcium alginate encapsulated Ni/Fe nanoparticles beads for simultaneous removal of Cu (II) and monochlorobenzene. J Colloid Interface Sci 447:85–91
Gok C, Aytas S (2009) Biosorption of uranium (VI) from aqueous solution using calcium alginate beads. J Hazard Mater 168:369–375
Torres E, Mata YN, Blazquez ML, Munoz JA, Gonzalez F, Ballester A (2005) Gold and silver uptake and nanoprecipitation on calcium alginate beads. Langmuir 21:7951–7958
Dalmoro A, Sitenkov AY, Cascone S, Lamberti G, Barba AA, Moustafine RI (2017) Hydrophilic drug encapsulation in shell-core microcarriers by two stage polyelectrolyte complexation method. Int J Pharm 518:50–58
Zhang YH, Lin XY, Hu SH, Zhang X, Luo XG (2016) Core-shell zeolite@Alg–Ca particles for removal of strontium from aqueous solutions. RSC Adv 6:73959–73973
Sikder MT, Mihara Y, Islam MS, Saito T, Tanaka S, Kurasaki M (2014) Preparation and characterization of chitosan–caboxymethyl-β-cyclodextrin entrapped nanozero-valent iron composite for Cu (II) and Cr(IV) removal from wastewate. Chem Eng J 236:378–387
Fiedor JN, Bostick WD, Jarabek RJ, Farrell J (1998) Understanding the mechanism of uranium removal from groundwater by zero-valent iron using X-ray photoelectron spectroscopy. Environ Sci Technol 321:466–1473
Xi YF, Sun ZM, Hreid T, Ayoko GA, Frost RL (2014) Bisphenol A degradation enhanced by air bubbles via advanced oxidation using in situ generated ferrous ions from nano zero-valent iron/palygorskite composite materials. Chem Eng J 247:66–74
Liu HB, Chen TH, Zou XH, Xie QQ, Qing CS, Chen D, Frost RL (2013) Removal of phosphorus using NZVI derived from reducing natural goethite. Chem Eng J 234:80–87
Xi YF, Mallavarapu M, Naidu R (2010) Reduction and adsorption of Pb2+ in aqueous solution by nano-zero-valent iron-A SEM, TEM and XPS study. Mater Res Bull 45:1361–1367
Yan W, Herzing AA, Kiely CJ, Zhang WX (2010) Nanoscale zero-valent iron (nZVI): aspects of the core-shell structure and reactions with inorganic species in water. J Contam Hydrol 118:96–104
Zhang ZB, Liu J, Cao XH, Luo XP, Hua R, Liu Y, Liu YH (2015) Comparison of U (VI) adsorption onto nanoscale zero-valent iron and red soil in the presence of U (VI)–CO3/Ca–U(VI)–CO3 complexes. J Hazard Mater 300:633–642
Kazy SK, D’Souza SF, Sar P (2009) Uranium and thorium sequestration by a Pseudomonas sp.: mechanism and chemical characterization. J Hazard Mater 163:65–72
Qian LP, Ma MH, Cheng DH (2014) The effect of water chemistry on adsorption and desorption of U (VI) on nano-alumina. J Mol Liq 197:295–300
Zulfikar MA, Afrita S, Wahyuningrum D, Ledyastuti M (2016) Preparation of Fe3O4-chitosan hybrid nano-particles used for humic acid adsorption. Environ Nanotechnol Monit Manag 6:64–75
Ngah WSW, Fatinathan S (2010) Adsorption characterization of Pb(II) and Cu(II) ions onto chitosan-tripolyphosphate beads: kinetic, equilibrium and thermodynamic studies. J Environ Manag 91:958–969
Tomar V, Prasad S, Kumar D (2013) Adsorptive removal of fluoride from water samples using Zr-Mn composite material. Microchem J 111:116–124
Zhang GK, He ZL, Xu W (2012) A low-cost and high efficient zirconium-modified-Na-attapulgite adsorbent for fluoride removal from aqueous solutions. Chem Eng J 183:315–324
Sikder MT, Tanaka S, Saito T, Kurasaki M (2014) Application of zerovalent iron impregnated chitosan-caboxymethyl-β-cyclodextrin composite beads as arsenic sorbent. J Environ Chem Eng 2:370–376
Zhou LM, Jin JY, Liu ZR, Liang XZ, Shang C (2011) Adsorption of acid dyes from aqueous solutions by the ethylenediamine-modified magnetic chitosan nanoparticles. J Hazard Mater 185:1045–1052
İnan S, Altaş Y (2011) Preparation of zirconium-manganese oxide/polyacrylonitrile (Zr-Mn oxide/PAN) composite spheres and the investigation of Sr (II) sorption by experimental design. Chem Eng J 168:1263–1271
Wang J, Chen T, Li S, Yue Z, Jin J, He G, Zhang H (2012) Biosorption of copper (II) from aqueous solutions with rape straw. Geomicrobiol J 29:250–254
Bulut E, Ozacar M, Sßengil IA (2008) Adsorption of malachite green onto bentonite: equilibrium and kinetic studies and process design. Microporous Mesoporous Mater 115:234–246
Hao YM, Chen M, Hu ZB (2010) Effective removal of Cu (II) ions from aqueous solution by amino-functionalized magnetic nanoparticles. J Hazard Mater 184:392–399
Volesky B (2003) Biosorption process simulation tools. Hydrometallurgy 71:179–190
Duan SX, Liu X, Wang Y, Shao D, Alharbi NS, Alsaedi A, Li J (2016) Highly efficient entrapment of U (VI) by using porous magnetic Ni0.6Fe2.4O4 micro-particles as the adsorbent. J Taiwan Inst Chem E 65:367–377
Abdi S, Nasiri M, Mesbahi A, Khani MH (2017) Investigation of uranium (VI) adsorption by polypyrrole. J Hazard Mater 332:132–139
Tian G, Geng J, Jin Y, Wang C, Li S, Chen Z, Li S (2011) Sorption of uranium (VI) using oxime-grafted ordered mesoporous carbon CMK-5. J Hazard Mater 190:442–450
Morsy AMA (2015) Adsorptive removal of uranium ions from liquid waste solutions by phosphorylated chitosan. Environ Technol Innov 4:299–310
Hazer O, Kartal S (2009) Synthesis of a novel chelating resin for the separation and preconcentration of uranium (VI) and its spectrophotometric determination. Anal Sci 25:547–551
Zhou L, Shang C, Liu Z, Huang G, Adesina AA (2012) Selective adsorption of uranium (VI) from aqueous solutions using the ion-imprinted magnetic chitosan resins. J Colloid Interface Sci 366:165–172
Tan L, Wang J, Liu Q, Sun Y, Zhang H, Wang Y, Song D (2015) Facile preparation of oxine functionalized magnetic Fe3O4 particles for enhanced uranium (VI) adsorption. Colloid Surface A 466:85–91
Wang G, Liu J, Wang X, Xie Z, Deng N (2009) Adsorption of uranium (VI) from aqueous solution onto cross-linked chitosan. J Hazard Mater 168:1053–1058
Acknowledgements
This work was sponsored the National Key Scientific Projects for Decommissioning of Nuclear Facilities and Radioactive Waste Management(14zg6101), Longshan academic talent research support plan of Southwest University of Science and Technology (17LZX302) and supported by Postgraduate Innovation Fund Project by Southwest University of Science and Technology(17ycx004). The authors would like to thank the Engineering Research Center of Biomass Materials, Ministry of Education, Southwest University of Science and Technology (14tdsc02).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hu, S., Lin, X., Zhang, Y. et al. Preparation of Ca-alginate coated nZVI core shell beads for uranium (VI) removal from aqueous solution. J Radioanal Nucl Chem 314, 2405–2416 (2017). https://doi.org/10.1007/s10967-017-5529-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-017-5529-y