Abstract
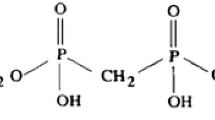
This paper describes a new analyte extraction technique using polymer ligand film (PLF). PLFs were synthesized to perform direct sorption of analytes onto its surface for direct counting using alpha spectroscopy. The main focus of the new technique is to shorten and simplify the procedure for chemically isolating radionuclides for determination through a radiometric technique. 4′(5′)-di-t-butylcyclohexano 18-crown-6 (DtBuCH18C6) and 2-ethylhexylphosphonic acid (HEH[EHP]) were examined for plutonium extraction. Di(2-ethyl hexyl) phosphoric acid (HDEHP) were examined for plutonium and uranium extraction. DtBuCH18C6 and HEH[EHP] were not effective in plutonium extraction. HDEHP PLFs were effective for plutonium but not for uranium.







Similar content being viewed by others
References
Plionis A, Haas D, Landsberger S, Brooks G (2008) A robust, field-deployable method for the electrodeposition of actinides. J Radioanal Nucl Chem 276:369–373. doi:10.1007/s10967-008-0514-0
Maxwell SL, Culligan BK, Kelsey-Wall A, Shaw PJ (2011) Rapid determination of actinides in emergency food samples. J Radioanal Nucl Chem 292:339–347. doi:10.1007/s10967-011-1411-5
Maxwell SL, Culligan BK, Hutchison JB, Spencer RB (2013) Rapid fusion method for determination of actinides in fecal samples. J Radioanal Nucl Chem 298:1533–1542. doi:10.1007/s10967-013-2541-8
Bari A, Khan AJ, Semkow TM et al (2011) Rapid screening of radioactivity in food for emergency response. Appl Radiat Isot 69:834–843. doi:10.1016/j.apradiso.2011.02.022
Rim JH, Gonzales ER, Armenta CE et al (2013) Developing and evaluating di(2-ethylhexyl) orthophosphoric acid (HDEHP) based polymer ligand film (PLF) for plutonium extraction. J Radioanal Nucl Chem 296:1099–1103. doi:10.1007/s10967-012-2266-0
Gonzáles ER, Peterson DS (2009) Rapid radiochemical sample preparation for alpha spectrometry using polymer ligand films. J Radioanal Nucl Chem 282:543–547. doi:10.1007/s10967-009-0218-0
Gonzáles ER, Klingensmith AL, Peterson DS (2011) Rapid separation and extraction of radioactive analytes onto filters and surfaces. Proc Radiochem Suppl Radiochim Acta 1:194–200. doi:10.1524/rcpr.2011.0035
Hanson SK, Mueller AH, Oldham WJ Jr (2014) Kläui ligand thin films for rapid plutonium analysis by alpha spectrometry. Anal Chem 86:1153–1159. doi:10.1021/ac402997e
Oldham WJ, Dry DE, Mueller AH (2009) Synthesis of functional monolayer surfaces for rapid radiometric determination of plutonium. J Radioanal Nucl Chem 282:585–589. doi:10.1007/s10967-009-0243-z
Koulouridakis PE, Kallithrakas-Kontos NG (2004) Selective mercury determination after membrane complexation and total reflection X-ray fluorescence analysis. Anal Chem 76:4315–4319. doi:10.1021/ac049780a
Surbeck (2000) Alpha spectrometry sample preparation using selectively adsorbing thin films. Appl Radiat Isot 53:97–100
Graul TW, Li M, Schlenoff JB (1999) Ion exchange in ultrathin films. J Phys Chem B 103:2718–2723. doi:10.1021/jp983049o
Wang L, Paimin R, Cattrall RW et al (2000) The extraction of cadmium(II) and copper(II) from hydrochloric acid solutions using an Aliquat 336/PVC membrane. J Membr Sci 176:105–111. doi:10.1016/S0376-7388(00)00436-1
McAlister D, Horwitz P (2011) Lanthanide separations
Rim J (2013) Development of novel method for rapid extract of radionuclides from solution using polymer ligand film. Pennsylvania State University, State College
Ganguly BN (1990) Spectroscopic investigation of uranium complexation in the reversed micellar system HDEHP—n-heptane—water. J Photochem Photobiol Chem 51:401–409. doi:10.1016/1010-6030(90)87074-L
Svantesson I, Persson G, Hagström I, Liljenzin JO (1980) Distribution ratios and empirical equations for the extraction of elements in Purex high level waste solution—II: HDEHP. J Inorg Nucl Chem 42:1037–1043. doi:10.1016/0022-1902(80)80397-6
Acknowledgments
This research was performed with the support of the U.S. Departments of Energy Office of Nuclear Nonproliferation Research and Development and U.S. Department of Defense’s Defense Threat Reduction Agency. The authors also gratefully acknowledge the support from the Nuclear Forensics Graduate Fellowship Program which is sponsored by the U.S. Department of Homeland Security’s Domestic Nuclear Detection Office and the U.S. Department of Defense’s Defense Threat Reduction Agency. Los Alamos National Laboratory is operated by Los Alamos National Security, LLC for the U.S. Department of Energy under contract number DE-AC52-06NA25396. This document had been reviewed and assigned publication number: LA-UR-14-27688 Version 4.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rim, J.H., Peterson, D.S., Armenta, C.E. et al. Evaluating ligands for use in polymer ligand film (PLF) for plutonium and uranium extraction. J Radioanal Nucl Chem 305, 193–198 (2015). https://doi.org/10.1007/s10967-015-4118-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-015-4118-1