Abstract
Two novel maltol-based ionic liquids, namely [A336][Mal] and [C101][Mal], were synthesized as potential extracting agents for radionuclides from water. These two room temperature task-specific ionic liquids could be easily prepared by anion metathesis starting from commercially available materials. The isolated compounds were characterized by standard analytical methods. Their application as extraction agent for Unat., 234Th, 210Pb, 210Bi, 210Po and 226Ra was elucidated by liquid–liquid extraction and scintillation counting. Uranium was totally extracted by both ionic liquids over a broad pH range (2–8), while the other radionuclides were removed with differing efficacies depending on the respective pH value.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In general, ionic liquids are defined as salts with melting points below 100 °C. They are characterized by a low vapor pressure, high decomposition temperatures or electrical stability [1]. Their physicochemical properties including their hydrophobicity can be fine-tuned by specific modifications of either the cation or anion. Task-specific ionic liquids (TSILs) allow new possibilities such as heavy metal extraction by anchoring functional groups. Heavy metal ions such as Cd2+ and Hg2+ are known to be harmful and TSILs are a promising approach for the metal removal by biphasic separation. Previous liquid–liquid extraction strategies for uranium revealed high efficacies in the case of [A336][TS] and [A336][SCN] (Fig. 1) from natural mineral water. In addition, these ionic liquids were investigated for a broad range of different metal ions and promising extraction efficacies were observed for mercury and platinum [2]. Another interesting feature beside their metal extraction potential is their high distribution coefficients >1,000 compared to Ca(II) and Mg(II) [3].
Uranium is prevalent in the environment and bioavailable as uranyl ion. By the general public a few μg/day uranium are taken up by ingestion of foodstuffs and of drinking water [4]. The target organ in the human body are the kidneys and nephritis is the primary effect of uranium intoxication. Epidemiological studies in Canada, Sweden and Finland saw some minor signs of adverse renal affects but on the other hand no severe cytotoxic effects were observed even with relatively high exposures (median concentration 25 μg/L, interquartile range of 5–148 μg/L, maximum conc. of 1,500 μg/L) [5]. So the World Health Organization set a guideline value for uranium of 30 µg/L drinking water in 2012 [6]. While higher uranium levels are very rare in public water supplies, concentrations up to 1.2 mg/L of uranium were found in water samples from private wells in Europe and USA [7], [8], [9].
Aside from radiation protection issues, the recovery of uranium, thorium and lanthanides from nuclear wastes is increasingly important [10]. Recently, tricaprylmethylammonium phthalate showed promising extraction rates of U(VI), Th(IV) and Fe(III) up to 99 %. Moreover, uranium was well separated at pH 0.1 from La(III), Y(III) and Nd(III) [11]. Tetradecyltrihexylphosphonium chloride and tricaprylmethylammonium chloride are hydrophobic ionic liquids and therefore possess interesting features for metal removal by liquid–liquid extraction. Tricaprylmethylammonium chloride itself showed promising results for the extraction of 99Mo and 181W [12] while tetradecyltrihexylphosphonium chloride has been developed for the selective extraction of cobalt in the presence of nickel [13]. Maltol is in extensive research over the last decades, due to its complexation ability to a broad range of metal ions. It is used in food industry as flavour enhancer and is characterized by its preferable toxicity profile and high bioavailability. Maltol is utilized due to its highly beneficial chemical and biological properties. In addition, it is commercially available even in large scale and is well known for its synthetic versatility. Due to environmental benign character and cost efficacy, ionic liquids based on this organic scaffold might be a promising alternative to the recently reported TSIL [A336][TS] and a good starting point for the development of ionic liquids as extracting agent for uranium [14]. Previously, 4-hydroxypyrylium-based ionic liquids showed interesting features as potential media for the immobilisation of catalysts [15].
Herein we report the synthesis of two new TSILs, tricaprylmethylammonium maltolate and tetradecyltrihexylphosphonium maltolate (Fig. 2) for the extraction of heavy metals from waste water. The presented compounds are the first step in the development of maltolato-based TSILs with optimized physicochemical parameters for the extraction of heavy metals. The respective maltolate salts were prepared by anion metathesis and characterized by standard analytical methods such as 1H and 13C NMR spectroscopy, IR and UV–vis spectroscopy. The total chloride content and the total organic carbon (TOC) values of these ionic liquids were determined. The extraction efficacies of two ionic liquids with regard to the naturally occurring radionuclides 238U and 234U, 234Th, 226Ra, 210Pb and its progenies 210Bi and 210Po were investigated.
Materials and methods
3-Hydroxy-2-methyl-4(1H)-pyrone (maltol, 99 %) and Cyphos IL 101 (tetradecyltrihexylphosphonium chloride, 95 %) were purchased from Sigma Aldrich. Potassium hydroxide (85 %, extra pure) and Aliquat 336® (mixture of quaternary ammonium compounds, tri-(C8-10)-alkylmethyl, chlorides, >75 %) were obtained from Acros. Utilized solvents were of HPLC grade and used without further purification. 1H and 13C NMR spectra were recorded on a Bruker Avance IIITM spectrometer in DMSO-d6 at 298 K using standard pulse programs at 500.10 (1H) and 125.76 (13C) MHz. UV–Vis spectra were performed on an Agilent 8453 spectrophotometer in the range of 200–1,000 nm in methanol. ATR-IR spectra were measured with a Bruker Vertex 70 Fourier transform IR spectrometer. TOC measurements were performed on a TOC-V CHP analyser (Shimadzu) and density measurements with a calibrated glass pycnometer (Klaus Hofmann). Viscosity measurements were conducted using a Kinexus rheometer (plate/plate method; temperature range: 298–323 K; test time: 2 min). The water content of [A336][Mal] and [C101][Mal] was determined utilizing a Mettler Toledo DL39 Karl Fischer (KF) coulometer; each ionic liquid was measured in triplicates. In-house standard solutions of different radionuclides were used for the extraction experiments. The uranium standard contained 27.7 μg Unat. in 10 μL diluted HNO3, corresponding to an activity of 682 mBq (238U and 234U), as well as 341 mBq 234Th (half-life 24.1 days) in radioactive equilibrium with 238U. The 210Pb (half-life 22.3 years) standard solution had an activity concentration of 580 mBq/10 μL. The progenies 210Bi (half-life 5 day) and 210Po (half-life 138.4 days) are in radioactive equilibrium with 210Pb, which means that each of them have an activity concentration of 580 mBq/10 μL. For radium extraction we used 2 mL of the reference material IAEA-431 (29.5.2001) containing 50 mBq 226Ra. Ingrown 222Rn left the sample during shaking with the IL for 226Ra extraction; as the activity measurements were always done immediately after the extraction step the in-growth of 222Rn and its short lived progenies was prevented.
Synthesis of ionic liquids
[A336][Mal]: To a solution of maltol (100 mg, 0.79 mmol) in methanol (5 mL) potassium hydroxide (49 mg, 0.88 mmol) was added in one portion and the solution was stirred for 10 min at room temperature. Aliquat 336® (106 mg, 0.26 mmol) in methanol (5 mL) was added and the reaction mixture was stirred for further 4 h. After complete evaporation, the residue was extracted with ethyl acetate/water (7 mL/4 mL). The separated organic phase was dried over Na2SO4, evaporated under reduced pressure and dried in vacuo, yielding an orange viscous oil. Yield: 122 mg (95 %). 1H NMR (500.10 MHz, DMSO-d6): δ = 7.71 (d, 3 J = 6 Hz, 1H, H6), 6.04 (d, 3 J = 6 Hz, 1H, H5) 3.15–3.25 (m, 6H, N-CH2), 2.96 (s, 3H, N-CH3), 2.19 (s, 3H, -CH3), 1.56–1.66 (m, 6H, -CH2-), 1.20–1.37 (m, 30H, -CH2-), 0.83–0.91 (m, 9H, -CH3). 13C{1H} NMR (125.76 MHz, DMSO-d6): δ 173.2, 155.0, 149.7, 143.6, 114.0, 61.2, 61.0, 48.0, 31.8, 31.6, 29.4, 29.3, 29.2, 28.9, 28.9, 26.3, 26.2, 22.6, 22.5, 21.8, 14.4. IR (ATR, selected bands, νmax): 2925, 2857, 1620, 1463, 1377, 1260, 1196, 1073, 916, 848, 661, 628 cm−1. UV–Vis in MeOH, λ, nm (ε, M−1 cm−1): 215 (10690), 275 (8161).
[C101][Mal]: To a solution of maltol (100 mg, 0.79 mmol) in methanol (5 mL) potassium hydroxide (49 mg, 0.88 mmol) was added in one portion and the solution was stirred for 10 min at room temperature. Tetradecyltrihexylphosphonium chloride (135 mg, 0.26 mmol) in methanol (5 mL) was added and the reaction mixture was stirred for further 4 h. After complete evaporation, the residue was extracted with ethyl acetate/water (7 mL/4 mL). The separated organic phase was dried over Na2SO4, evaporated under reduced pressure and dried in vacuo, yielding an orange viscous oil. Yield: 149 mg (94 %). 1H NMR (500.10 MHz, DMSO-d6): δ = 7.91 (d, 3 J = 6 Hz, 1H, H6), 6.21 (d, 3 J = 6 Hz, 1H, H5) 2.22 (s, 3H, -CH3), 2.15–2.21(m, 8H, -CH2-), 1.43–1.53 (m, 8H, -CH2-), 1.34–1.43 (m, 9H, -CH2-), 1.21–1.34 (m, 36H, -CH2-), 0.83–0.92 (m, 12H, -CH3). 13C{1H} NMR (125.76 MHz, DMSO-d6): δ 173.0, 155.1, 149.6, 143.4, 116.8, 114.0, 31.8, 30.9, 30.5, 30.4, 30.3, 30.2, 29.5, 29.5, 29.4, 29.2, 29.1, 28.6, 22.6, 22.3, 21.1, 21.0, 18.3, 18.2, 17.9, 17.8, 14.4, 14.3. IR (ATR, selected bands, νmax): 3251, 3061, 2923, 2854, 1653, 1629, 1458, 1369, 1255, 1223, 1197, 1077, 1022, 919, 848, 688 cm−1. UV–Vis in MeOH, λ, nm (ε, M−1 cm−1): 215 (16380), 277 (12392).
Liquid–liquid extraction experiments
Extractions were performed by weighting selected amounts (100–200 mg) of ionic liquids in 15 mL polypropylene tubes and addition of about 10 mL of aqueous solution containing a known amount of Unat., 210Pb, or 226Ra (the pH values had been adjusted by addition of HNO3 or NaOH). Samples were shaken overnight at 100 rpm and subsequently centrifuged for 30 min at 3,500 rpm [the relative centrifugal force (RCF) is 1646] for phase separation. The yield of the various extractions was determined by measuring the remaining activity concentration of the respective radionuclides in the aqueous phase by liquid scintillation counting (LSC). The advantage of this method is twofold: an aliquot of the aqueous solution can be measured directly without further sample treatment, and α- and β-emitters present in the same sample can be measured simultaneously. The poor energy resolution in the α-spectrum is of no relevance, as the 234U/238U ratio is not altered by the extraction in any way.
All extraction experiments were done at least twofold with satisfying agreement. The maximum difference of the extraction yields from an aqueous solution (at a given pH value) measured for two different batches of the same IL were 20 %, comprising also the 1σ-counting uncertainties of 5–10 %. These relatively high counting uncertainties are due to the fact that high extraction yields correspond to low activities left in the aqueous phase, which is then measured. By extending the counting time the uncertainties could be reduced.
Instrumental setup
For the LSC measurement an aliquot of the aqueous phase (usually 3 mL) was transferred to a 20 mL vial and mixed with the scintillation cocktail HiSafe III™. After cooling, the activity concentrations of the respective radionuclides were measured with a Quantulus™ 1220 low-level liquid scintillation counter (Wallac Oy, Finland, now Perkin Elmer). Counting times were 300 min or less, depending on the activity of the respective samples. As the uranium solution contains also the β-emitter 234Th (+ 234Pa), and 210Pb is in radioactive equilibrium with its progenies 210Bi (also a β-emitter) and 210Po (α-emitter), pulse shape analysis was used to differ between α- and β-counts (light pulses following α-emission have a longer decay time than pulses following β-emission and can therefore be distinguished electronically).
Leaching and chloride content measurements
Leaching of the ionic liquids into the water phase was tested the following way: 100 mg of IL were shaken with double distilled water (5 mL) for 24 h. 1.2 mL of the solution were diluted with 10.8 mL double distilled water. The samples were acidified to pH 2 with HCl and purged with carrier gas for 5 min prior to combustion in the TOC analyser. The purities of the tested ionic liquids were quantified by their residual chloride ions. The chloride content was determined by dissolving the sample in 10 mL ethanol/water (1:1), acidified with HNO3 (half concentrated) and potentiometric titrated using AgNO3 as titrant based on two independent experiments.
Results and discussion
Synthesis and characterization
The two maltol-based ionic liquids were prepared by anion metathesis, which is a fast and sustainable standard strategy in literature [16]. Therefore, maltol was deprotonated with an excess of potassium hydroxide to ensure it reacts quantitatively and checked by 1H NMR. Afterwards, either Aliquat 336® (a mixture of quaternary ammonium compounds) or Cyphos IL101 was added and after aqueous work up the desired products were isolated in high yield (>90 %) and sufficient purity. An excess of maltol was necessary in both syntheses due to the high solubility of maltolate in water. The isolated ionic liquids were characterized by standard analytical methods (1H and 13C NMR, infrared and UV–Vis spectroscopy, density, viscosity, water and chloride content).
Both synthesized compounds were isolated as highly viscous oils at room temperature and the formation of the 1:1 salts was confirmed by 1H NMR spectroscopy (Figure S1 and S2). The chloride content of the ionic liquids [A336][Mal] and [C101][Mal] has been found to be 1.5 and 1.7 %, respectively, by argentometric titration (Table 1).
Physicochemical properties
The physiochemical properties of novel ionic liquids such as melting point, density, miscibility or viscosity, define and limit the potential applications. The synthesized maltolate-based ionic liquids were found to be soluble in common organic solvents such as chloroform and ethanol, but insoluble in water. The leaching in water was determined by TOC experiments for the two ionic liquids with values of dissolved carbon of 3,800 mg/L for [A336][Mal] and 3,470 mg/L for [C101][Mal] (Table 1), respectively. This result can be explained by the high solubility of the maltolate in aqueous systems. The viscosities, determined by a temperature gradient from 298 to 323 K, are presented in Fig. 3 where [A336][Mal] shows higher viscosity than the corresponding phosphonium ionic liquid which is in good accordance to literature [17]. The different behavior might be explained by the varying chain lengths of the two applied cations. The water content of [A336][Mal] and [C101][Mal] was determined by Karl Fischer coulometry with values of 1.3 % (w/w), determined by three independent experiments. This is an important feature because water influences the physical behavior of ionic liquids [18]. Room-temperature ionic liquids can be used without further dilution for liquid–liquid extraction. It has been reported that ionic liquids consisting of quaternary ammonium and phosphonium salts do mostly have densities less than 1 g/cm3 [19] [20], which is in good agreement with the determined densities of [A336][Mal] and [C101][Mal] of 0.98 g/cm3 at 298 K (see Table 1).
Extracting efficiency
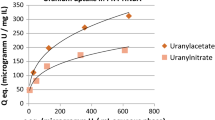
Due to the known high affinity of the maltolate to a broad range of different metal ions, extraction experiments with a series of radionuclides were performed to investigate potential applications. It was found that uranium was quantitatively extracted by [A336][Mal] and [C101][Mal] (Fig. 4) and the initial pH during the experiments showed no influence on the extraction rate. This can be explained by the HSAB principle, because the uranyl cation possesses a high affinity towards the O,O coordination motif of the maltolate backbone. It should be kept in mind that U(VI) is present in different species depending on the pH value of the solution. For example, at pH > 2.5 UO2(OH)+, (UO2)2(OH)2 2+ and (UO2)3(OH)5 + species are predominant whereas at pH < 2.5 UO2 2+ is prevalent [21]. However, although the solubility of U(VI) is higher under acidic conditions, it was extracted quantitatively from all samples [21]. 234Th is a progeny of 238U and in the case of [C101][Mal] the extraction of thorium decreased at higher pH which could be explained by lower affinity of 234Th(OH)2 2+ and other hydrolysis products to this IL compared to 234Th4+ present at lower pH values. In contrast, best extraction rate for 234Th with [A336][Mal] could be found at pH 6. 210Pb was removed less than 20 % of either [A336][Mal] or [C101][Mal] in all experiments and the pH value had no significant impact on the elimination. Up to 80 % of 210Po was removed at pH 2–6 with [A336][Mal], whereas [C101][Mal] was slightly less effective at pH > 4. Interestingly, at pH values ≥ 6, the extraction rate for 210Po decreased significantly for both compounds. Similar extraction data was observed for the 210Bi(III) removal of [A336][Mal] and [C101][Mal] with a maximum of about 40 % at pH 8. 226Ra was not extracted by both maltol-derived ionic liquids. At pH values higher than 6, only uranium could be completely eliminated from the radioactive solution.
Conclusion
Herein, we report the straight-forward and cost-saving synthesis of two new maltol-based ionic liquids with tricaprylmethylammonium and tetradecyltrihexylphosphonium as counterions from commercially available precursors by anion metathesis. The obtained compounds were characterized by standard analytical methods and the physicochemical properties were determined. Although a high leaching of the maltol-based ionic liquids was observed, the extraction experiments with different radionuclides were very promising. The extraction of uranium showed a quantitative efficiency for [A336][Mal] and [C101][Mal] over the investigated pH range (2–8), therefore, this method represents a suitable way for uranium removal. Additionally, 210Po and 234Th were well extracted; whereas no or only low removal of either 226Ra, 210Bi or 210Pb was observed. Back extraction experiments with different stripping agents are ongoing.
Overall, these investigations are the first step for the development of maltol-based ionic liquids with high heavy metal extraction rates and further synthetic modifications as well as stripping experiments towards their potential applications are ongoing.
References
Wasserscheid P (2006) Nature 439:797–797
Fischer L, Falta T, Koellensperger G, Stojanovic A, Kogelnig D, Galanski M, Krachler R, Keppler BK, Hann S (2011) Water Res 45:4601–4614
Srncik M, Kogelnig D, Stojanovic A, Koerner W, Krachler R, Wallner G (2009) Appl Radiat Isot 67:2146–2149
Fisenne IM, Perry PM, Decker KM, Keller HW (1987) Health Phys 53:357–363
Kurttio P, Harmoinen A, Saha H, Salonen L, Karpas Z, Komulainen H, Auvinen A (2006) Am J Kidney Dis 47:972–982
World Health Organization (2012) WHO/SDE/WSH/03.04/118/Rev/1
Selden AI, Lundholm C, Edlund B, Hogdahl C, Ek BM, Bergstrom BE, Ohlson CG (2009) Environ Res 109:486–494
Orloff KG, Mistry K, Charp P, Metcalf S, Marino R, Shelly T, Melaro E, Donohoe AM, Jones RL (2004) Environ Res 94:319–326
Hakonson-Hayes AC, Fresquez PR, Whicker FW (2002) J Environ Radioact 59:29–40
Raju CSK, Subramanian MS (2007) J Hazard Mater 145:315–322
Biswas S, Rupawate VH, Roy SB, Sahu M (2014) J Radioanal Nucl Chem 300:853–858
Yokokita T, Kasamatsu Y, Ooe K, Yoshimura T, Takahashi N, Komori Y, Shinohara A (2014) J Radioanal Nucl Chem 301:751–756
Wellens S, Goovaerts R, Möller C, Luyten J, Thijs B, Binnemans K (2014) Green Chem 15:3160
Liboiron BD, Thompson KH, Hanson GR, Lam E, Aebischer N, Orvig C (2005) J Am Chem Soc 127:5104–5115
Pernak J, Swierczynska A, Kot M, Walkiewicz F, Maciejewski H (2011) Tetrahedron Lett 52:4342–4345
Kogelnig D, Stojanovic A, Galanski M, Groessl M, Jirsa F, Krachler R, Keppler BK (2008) Tetrahedron Lett 49:2782–2785
Stojanovic A, Kogelnig D, Fischer L, Hann S, Galanski M, Groessl M, Krachler R, Keppler BK (2010) Aust J Chem 63:511–524
Martins VL, Nicolau BG, Urahata SM, Ribeiro MCC, Torresi RM (2013) J Phys Chem B 117:8782–8792
Fraser KJ, Macfarlane DR (2009) Aust J Chem 62:309–321
Kulkarni PS, Branco LC, Crespo JG, Nunes MC, Raymundo A Afonso CaM (2007) Chem Eur J 13:8478–8488
White C, Gadd GM (1990) J Chem Technol Biotechnol 49:331–343
Acknowledgments
The authors thank the Microanalytical Laboratory, Institute of Physical Chemistry, University of Vienna, for the chloride content measurements and Gerhard Schleining, University of Natural Resources and Applied Life Science, Department of Food Science and Technology, for using the viscometer. The study was partly funded by the FWF Austrian Science Fund (Project number P 24676-N19).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Platzer, S., Sap, O., Leyma, R. et al. Extraction of natural radionuclides from aqueous solutions by novel maltolate-based task-specific ionic liquids. J Radioanal Nucl Chem 303, 2483–2488 (2015). https://doi.org/10.1007/s10967-014-3782-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-014-3782-x