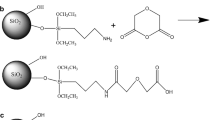
Abstract
In this study, the adsorption process was examined by various isotherm models Langmuir, Freundlich and Dubinin–Radushkevich and equilibrium data were successfully described by Langmuir model. Adsorption thermodynamics of uranium (VI) on modified silica gel (SiAPMS-HL) has been studied within a temperature range from 293 to 333 K and the thermodynamic parameters, such as equilibrium constant (K D), standard free energy changes (ΔG°), standard enthalpy change (ΔH°) and standard entropy change (ΔS°), have been obtained. The desorption studies were conducted in batch system to investigate the kind, concentration and volume of the eluent.


Similar content being viewed by others
References
Spryskyy M, Kovalchuk I, Buszewski B (2010) The separation of uranium ions by natural and modified diatomite from aqueous soltion. J Hazard Mater 181:700–707
Takahashi A, Ueki Y, Igarashi S (1999) Homogeneous liquid–liquid extraction of uranium(VI) from acetate aqueous solution. Anal Chim Acta 387:71–75
Mellah A, Chegrouche S, Barkat M (2006) The removal uranium (VI) from aqueous solutions onto activated carbon: kinetic and thermodynamic investigations. J Colloid Interf Sci 296:434–441
Krestou A, Xenidis A, Panias D (2005) Mechanism of aqueous uranium(VI) uptake by hydroxyapatite. Miner Eng 17:373–381
Aydin FA, Soylak M (2010) Separation, preconcentration and inductively coupled plasma-mass spectrometric (ICP-MS) determination of thorium(iv), titanium(iv), iron(iii), lead (ii) and chromium(iii) on 2-nitroso-1-naphthol impregnated mci gel chp20p resin. J Hazard Mater 173:669–674
Rao TP, Metilda P, Gladis JM (2006) Preconcentration techniques for uranium(VI) and thorium(IV) prior to analytical determination: an overview. Talanta 68:1047–1064
Kulkarni PS, Mukhopadhyay S, Bellary MP, Ghosh SK (2002) Studies on membrane stability and recovery of uranium (VI) from aqueous solutions using a liquid emulsion membrane process. Hydrometallurgy 64:49–58
Hayworth HC, Ho WS, Burns WA, Norman Jr, Li N (1983) Extraction of uranium from wet process phosphoric acid by liquid membranes. Sep Sci Technol 18:493–552
Pabby AK, Sastre AM (2013) State-of-the-art review on hollow fibre contactor technology and membrane-based extraction processes. J Membr Sci 430:263–303
Roach JD, Zapien JH (2009) Inorganic ligand-modified, colloid-enhanced ultrafiltration: a novel method for removing uranium from aqueous solution. Water Res 43(18):4751–4759
Semião AJC, Rossiter HMA, Schäfer A (2011) Impact of organic matter and speciation on the behaviour of uranium in submerged ultrafiltration. J Membr Sci 348:174–180
Aytas S, Yurtlu M, Donat R (2009) Adsorption characteristic of U(VI) ion onto thermally activated bentonite. J Hazard Mater 172:667–674
Christids GE, Scott PW, Dunham AC (1997) Acid activation and bleaching capacity of bentonites from the islands of Milos and Chios, Aegean, Greece. Appl Clay Sci 12:329–347
Oshita K, Seo K, Sabarudin A, Oshima M, Takayanagi T, Motomizu S (2009) Adsorption behavior of uranium(VI) and other ionic species on cross-linked chitosan resins modified with chelating moieties. Talanta 79:1031–1035
Oshita K, Oshima M, Gao YH, Lee KH, Motomizu S (2003) Anal Chim Acta 480:239–249
Satoko Y, Koji O, Sabarudin A, Mitsuko O, Shoji M (2004) Bunseki Kagaku 53:1039–1043
Sabarudin A, Oshima M, Takayanagi T, Hakim L, Oshita K, Gao YH, Motomizu S (2007) Functionalization of chitosan with 3, 4-dihydroxybenzoic acid for the adsorption/collection of uranium in water samples and its determination by inductively coupled plasma-mass spectrometry. Anal Chim Acta 581:214–220
Wang GH, Liu JS, Wang XG, Xie ZY, Deng NS (2009) Adsorption of uranium(VI) from aqueous solution onto cross-linked chitosan. J Hazard Mater 168:1053–1058
Liu YH, Cao XH, Le ZG, Luo MB, Xu WY, Huang GL (2010) Selective adsorption of uranyl ion on ion-imprinted chitosan/PVA cross-linked hydrogel. Hydrometallurgy 104:150–155
Pang C, Liu YH, Cao XH, Hua R, Wang CX, Li CQ (2010) Adsorptive removal of uranium from aqueous solution using chitosan-coated attapulgite. J Radioanal Nucl Chem 286:185–193
Saleem M, Afzal M, Qadeer R, Hanif J (1992) Selective adsorption of uranium on activated charcoal from electrolytic aqueous solution. Sep Sci Technol 27:239–253
Kütahyali C, Eral M (2004) Selective adsorption of uranium from aqueous solutions using activated carbon prepared from charcoal by chemical activation. Sep Purif Technol 40:109–114
Morsy AMA, Hussein AEM (2011) Adsorption of uranium from crude phosphoric acid using activated carbon. J Radioanal Nucl Chem 288:341–346
Qadeer R, Hanif J (1994) Kinetics of uranium (VI) adsorption on activated charcoal from aqueous solutions. Radiochim Acta 65:259–263
Abbasi WA, Streat M (1994) Adsorption of uranium from aqueous solutions using activated carbon. Sep Sci Technol 29:1217–1230
Marzal P, Seco A, Gabaldon C, Ferrer J (1996) J Chem Technol Biotechnol 66:279–288
Silem A, Boualia A, Kada R, Mellah A (1992) Adsorption of organic matter from a wet phosphoric acid using activated carbon: batch-contact time study and linear driving force model. J Chem Eng 70:491–498
El Aamrani FZ, Duro L, de Pablo J, Bruno J (2002) Experimental study and modeling of the sorption of uranium(VI) onto olivine-rock. Appl Geochem 17:399–408
Parab H, Joshi S, Shenoy N, Verma R, Lali A, Sudersanan M (2005) Uranium removal from aqueous solution by coir pith: equilibrium and kinetic studies. Bioresour Technol 96:1241–1248
Chrisholm-Brause CJ, Berg JM, Little KM, Matzner RA, Morris DE (2004) Uranyl sorption by smectites: spectroscopic assessment of thermodynamic modelling. J Colloid Interface Sci 277:366–382
Payne TE, Davis JA, Lumpkin GR, Chisari R, Waite TD (2004) Surface complexation model of uranyl sorption on Georgia kaolinite. Appl Clay Sci 26:151–162
Missana T, Garcia-Gutierrez M, Alonso U (2004) Kinetics and irreversibility of cesium and uranium sorption onto bentonite colloids in a deep granitic environment. Appl Clay Sci 26:137–150
Chegrouche S, Mellah A, Telmoune S (1997) Removal of lanthanum from aqueous solutions by natural bentonite. Water Res 31(7):1733–1737
Kozar S, Bilinski H, Branica M, Schwugar M (1992) Adsorption of Cd(II) and Pb(II) on bentonite under estuarine and seawater conditions. J Sci Total Environ 121:203–216
Olguin MT, Rios MS, Acosta D, Bosch P, Bulbulian S (1997) UO2 2+ sorption on bentonite. J Radional Nucl Chem 218:65–69
Boualia A, Mellah A, Aissaoui TT, Menacer K, Silem A (1993) Adsorption of organic matter contained in industrial H3PO4 onto bentonite: batch-contact time and kinetic study. Appl Clay Sci 7:431–445
Mellah A, Chegrouche S (1997) The removal of zinc from aqueous solutions by natural bentonites. Water Res 31(3):621–629
Catalano JG, Brown GE Jr (2005) Uranyl adsorption onto montmorillonite: evaluation of binding sites and carbonate complexation. Geochim Cosmochim Acta 69:2995–3005
Kalin M, Wheeler WN, Meinrath G (2004) The removal of uranium from mining waste using algal/microbial biomass. J Environ Radioact 78:151–177
Yating L, Yunhai L, Xiaohong C, Rong H, Youqun W, Cui P, Ming H, Xiaoyan L (2011) Biosorption studies of uranium (VI) on cross-linked chitosan: isotherm, kinetic and thermodynamic aspects. J Radioanal Nucl Chem 290:231–239
Wan Ngah WS, Fatinathan S (2008) Adsorption of Cu (II) ions in aqueous solution using chitosan beads, chitosan–GLA beads and chitosan–alginate beads. Chem Eng J 143:62–72
Sadeghi S, Sheikhzadeh E (2008) Solid phase extraction using silica gel functionalized with Sulfasalazine for preconcentration of uranium(VI) ions from water samples. Microchim Acta 163:313–320
Karayel Incili G, Aycık GA (2014) Chemical modification of silica gel with synthesized Schiff base hydrazone derivative and application for preconcentration and separation of U(VI) ions from aqueous solutions. J Radioanal Nucl Chem. doi:10.1007/s10967-014-3151-9
El-Behery M, El-Twigry H (2007) Synthesis, magnetic, spectral and antimicrobial studies of Cu(II), Ni(II), Co(II), Fe(III) and UO2(II) complexes of a new Schiff base hydrazone derived from 7-chloro-4-hydrazinoquinoline. Spectrochim Acta A 66:28–36
Sadeghi S, Sheikhzadeh E (2009) Solid phase extraction using silica gel modified with murexide for preconcentration of uranium (VI) ions from water samples. J Hazard Mater 163:861–868
Aytas S, Turkozu DA, Gok C (2011) Biosorption of uranium (VI) by bi-functionalized low cost biocomposite adsorbent. Desalination 280:354–362
Hasany SM, Saeed MM, Ahmed M (2002) Sorption and thermodynamic behavior of zinc(II)–thiocyanate complexes onto polyurethane foam from acidic solutions. J Radioanal Nucl Chem 252:477–484
Saeed MM (2003) Adsorption profile and thermodynamic parameters of the preconcentration of Eu(III) on 2-thenoyltrifluoroacetone loaded polyurethane (PUR) foam. J Radioanal Nucl Chem 256:73–80
Yusan S, Akyil S (2008) Sorption of uranium(VI) from aqueous solutions by akaganeite. J Hazard Mater 160:388–395
Kütahyali C, Eral M (2010) Sorption studies of uranium and thorium on activated carbon prepared from olive stones: kinetic and thermodynamic aspects. J Nucl Mater 396:251–256
Humelnicu D, Valentina Dinu M, Dragan ES (2011) Adsorption characteristics of UO2 2+ and Th4+ ions from simulated radioactive solutions onto chitosan/clinoptilolite sorbents. J Hazard Mater 185:447–455
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karayel Incili, G., Aycik, G.A. Adsorption thermodynamic and desorption studies of U(VI) on modified silica gel (SiAPMS-HL). J Radioanal Nucl Chem 302, 79–85 (2014). https://doi.org/10.1007/s10967-014-3344-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-014-3344-2