Abstract
Fouling is challenging for the industrial implementation of continuous emulsion polymerisations. A requirement for the development of successful anti-fouling concepts is an in-depth understanding of the processes involved in fouling and the knowledge of the main influencing factors. In this work, an experiment-based strategy was developed for the quantification of fouling trends and in order to calculate the expected fouling intensities during the continuous emulsion polymerisation of vinyl acetate copolymers. These, then, can be correlated with the direct process parameters temperature, initiator and emulsifier content and comonomer ratio. The expected fouling tendency for formulations could be determined in the investigated range with an accuracy of ± 15% referred to experimental validations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As fouling is an important challenge for the chemical industry there are lots of patents regarding fouling reduction for different industrial polymerization systems or reaction processes. One widely used strategy is the fouling prevention by using additives during the process [1,2,3,4,5,6,7]. For example alkylphosphonate esters reduce the fouling during polymerisation of ethylene dichloride [3], polyoxyalkenes could be used for fouling reduction in solution polymerisation of ethylene co- and homopolymers [6] or alkyl and aryl phthalates could be used for the polymerisation of acrylates, methacrylate or acrylic acid. [7]. Another commonly used strategy in different fields of application is the coating of reactor surfaces [8,9,10,11]. Examples for this are monolayer coatings with polyvinyl alcohol and the disodium salt of bisphenol A for the polymerisation of vinyl halides, vinylidene halides or vinylidene monomers [8] or coatings with a water dispersion of alumina oxalyl bis(benzylidene)hydrazide [9] respectively aqueous selenous acid [10] for vinyl chloride polymerisation. Moreover there are some ideas for reducing fouling by special reactor concepts [12,13,14,15,16,17]. For example McFadden et al. [13] reported that fouling during continuous polymerization could be reduced by highly precise control of monomer streams and process parameters. Lowell et al. [14] investigated that special geometry changes could reduce fouling for gas phase polymerisation. Carvalho et al. [17] used at lab scale an oscillatory flow reactor for a fouling free continuous emulsion polymerization. For specialized applications there are ideas of adding comonomers to reduce fouling like for Kinetic Hydrate Inhibitor Formulations [18].
Besides fouling reduction or prevention there are some patents and publications regarding the optimized cleaning procedures for fouling intensive processes. For olefinic polymer deposits a cleaning procedure by circulating high boiling aromatic hydrocarbon solvents in the reactor and afterwards recovering them by flash separation is described [19]. Haruyama [20] published a cleaning strategy for mechanical cleaning of polymeric fouling. The polymerization is carried out in the gap between two tempered tubes built into each other, which can be tempered independently of each other for cleaning, so that the reaction gap is closed by material expansion or contraction and removes polymer residues [20]. Saikhwan et al. [21] investigated the swelling and cleaning behavior of non-cross-linked acrylate-styrene copolymers and reported temperature and pH conditions where cleaning is easily done.
In dependence of heating or cooling operations the prediction of heat exchanger fouling can be realized by the usage of learning algorithms [22].
Besides multiple publications on emulsion polymerization kinetics e.g. by Schork and Lu Fujun [23,24,25,26,27] and the influences of oxygen and process parameters on the nucleation and particle formation by Krishnan et al. [28] there are less publications discussing the mechanisms or reasons for fouling. Deglmann et al. [29] reported that the fouling processes in the radical polymerisation of N-vinyl pyrrolidone are mainly caused by transfer reactions leading to terminal double bonds which are crosslinked afterwards. This crosslinking leads to a significant increase in the molecular weight and is an important reason for deposit formation [29]. Neßlinger et al. [30] have investigated the fouling during solvent polymerisation of vinyl pyrrolidone. They discussed the influences of coatings of reactor surfaces on fouling masses and their possibility of fouling prevention [30]. Ekowati et al. [31] investigated the fouling on membranes during waste water treatment and reported that cationic polymers are causing fouling on reverse osmose membranes. They were able to predict the time dependent fouling masses and could distinguish between reversible fouling that could be removed by chemical cleaning and irreversible fouling that couldn’t be removed and influenced the efficiency of the membranes permanently [31].
Fouling processes during emulsion polymerisation of vinyl acetate are investigated in literature as well. There are different publications discussing this topic [17, 32,33,34]. Carvalho et al. [17] investigated the continuous emulsion copolymerisation of vinyl acetate and n-butyl acrylate in a tubular reactor. They reported that fouling during reaction caused problems and that could be solved by oscillating pulsed flow control and internal sieve plates in the reactor. The oscillating flow leads to short periods of turbulent flow control and prevents fouling while the internal sieve plates optimize radial mixing and reduce side reactions [17]. Moreover the fouling during emulsion copolymerisation of vinyl acetate and vinyl esters on heat-exchangers is investigated and compared to the fouling of commercial polyvinyl acetate dispersions on heated or cooled surfaces. In these publications the influences of temperature and surface modifications on fouling masses are discussed and it could be shown that higher temperatures lead to higher fouling masses. For surface modifications a general trend could not be observed as it is highly dependent on the other process parameters [32,33,34]. Hohlen et al. [33] investigated the differences between fouling of reactive emulsion polymerisation systems compared to the fouling of non-reactive polymer dispersions. Reactive systems cause more fouling than non-reactive so polymerisation fouling is the major part. The morphology of the deposits has its origin in the fouling process. Thus the dominant fouling process can be concluded from the fouling morphology [33].
For fouling quantification there are different strategies described in literature. Böttcher et al. [35] presented an in-line measurement technique for monitoring heat-transfer fouling masses during emulsion copolymerisation of butyl acrylate and methyl methacrylate using a quartz crystal microbalance with dissipation monitoring. They showed that there are two different pathways for heat-transfer fouling, one leading to thin fouling films which don’t grow further after being formed and the other leading to thick fouling which grows over time [35]. For fouling quantification in fouling intense processes it is commonly used practice to detect the changes in the weight of the reactor [36, 37]. In early stages of fouling this method is not as precise as a quartz crystal microbalance for example. For stronger fouling a quartz crystal microbalance does not work. The weight detection of the whole reactor ore parts of them is more robust.
Concluding all these publications there are lots of patents which optimize fouling behaviour in small, specialized applications but less literature which discusses scientific approaches to fouling processes. Most of the scientific publications are focussed on mechanistic influences or temperature effects so there are no publications addressing fouling behaviour to the set of process parameters that could be changed for the continuous emulsion polymerisation process of vinyl acetate copolymers. This work presents a strategy for evaluating the process and recipe dependent fouling behaviour in emulsion polymerisations and results in an experiment-based model for fouling prediction in the investigated system.
Experimental section
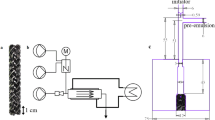
For the stirred tank in series concept the continuous production of polybutadiene and polyvinyl acetate derived latices is well established. In contrast tubular reactors are not well established in continuous emulsion polymerisation although the specific heat transfer capacity and thus the theoretic space time yield is much higher at lower costs than that of continuous stirred tank reactors. The main reason is the challenging fouling tendency of these products. In order to come forward with continuous emulsion polymerization experiments were carried out in a Fluitec ContiPlant LAB® tubular reactor with CSE-X static mixing elements (Fig. 1a). The half-shell reactor has a channel diameter of d = 12 mm filled with CSE-X static mixing elements and resulting volume of V = 45.5 mL. Temperature control was achieved by a cryostat of the type Julabo FP50 tempering the double jacket to a constant inlet temperature, closed loop temperature controlled at the jacket inlet (Fig. 1b).
Three solenoid-driven diaphragm metering pumps ProMinent® Gamma were used to deliver the dosing flows. The monomers (ProMinent gamma/4–1, stroke volume 0.13 mL/stroke, dosing flow 0.05 g/s) and the emulsifier solution containing poly(vinyl alcohol), Ascorbic acid and ammonium iron(III)sulphate (ProMinent gamma/5, stroke volume 0.16 mL/stroke, dosing flow 0.15 g/s) were pre-emulsified in a cylindrical CSTR equipped with a magnetic stirrer (d = 25.4 mm, V = 20 mL, dstirrer = 18 mm, 600 rpm) and the initiator flow containing tert-butyl hydroperoxide (ProMinent gamma/4, stroke volume 0.03 mL/stroke, dosing flow 0.01 g/s) was added 55 mm upstream and centered to the reactor inlet via a capillary nozzle (inner diameter 1 mm) (Fig. 1c).
All chemicals were used without further purifying. Vinyl acetate, vinyl neodecanoate and Mowiol 4–88 (molecular weight ~ 31 kDa, Degree of hydrolysis 88%) were purchased in technical grade from Wacker Chemie AG, Burghausen. Ascorbic acid and ammonium iron(III) sulphate dodecahydrate were purchased in analysis grade from Sigma Aldrich. tert-butyl hydroperoxide was purchased as an aqueous solution (c = 70%(w/w)) from Sigma Aldrich.
For the monomer feed a vinyl acetate-vinyl neodecanoate (VeoVa10®) comonomer stock system was used for emulsion polymerisation, with 0 – 20 wt% VeoVa10® based on total monomer content. The comonomer system is very advantageous for the investigation of fouling, as the addition of vinyl neodecanoate increases the hydrophobicity and lowers the glass transition temperature of the polymer, which favours film formation. Polymerisation were carried out with a monomer content of 23.8 wt%. Polyvinyl alcohol was used as an emulsifier in different weight proportions (5 – 15 wt% based on monomer). The initiation was carried out by a redox initiator system consisting of tert-butyl hydroperoxide, ascorbic acid and ammonium iron(III) sulphate dodecahydrate in a molar ratio of 1:1:0.03. The initiator weight proportions were varied between 0.1 – 2 wt% based on monomer. As a reaction time 120 min was chosen and fouling masses after this time compared. An overview of the used recipes is given in Table 1.
The influencing variables investigated were the reactor inlet temperature (5 °C—50 °C), the initiator content (0.1 wt%—2 wt% based on monomer), the emulsifier content (5 wt%—15 wt% based on monomer), and the monomer composition given as mole fraction of vinyl acetate xVac (0.8—1.0). The total monomer content was kept constant at 23.8 wt% within the scope of this work to obtain a valid model for a substance system that delivers reproducible results in the set-up used. The total monomer content was kept lower than in typical industrial processes as fouling would be much more occurring with 50 wt% total monomer content and influencing effects can’t be observed that good. So, a similar recipe with lower total monomer content was used for investigation in lab scale. For typical industrial applications with recipes with 50 wt% or more total monomer content similar trends can be expected but in the used lab scale plant changing monomer proportions would either massively influence the conversion of the reaction or require more profound changes to the reactor. A detailed overview of the conducted experiments is given in the supplementary information. The fouling in the reactor is growing on every surface in the reactor in similar amounts. So quantification is based on differential weighting of the static mixers as they are containing more than 95% of the inner reactor surfaces and effects of the reactor walls could be neglected. All fouling masses are given as fouling masses referenced to the weight of the static mixers in the reactor where the fouling mainly occurs (mgfouling/gmixer).
Conversions X(t) were calculated as the weight ratio between formed polymer wP and total initial monomer content wM (Eq. 1). The polymer content was measured by microwave gravimetry of the product dispersions.
Results
Fouling causes conversion changes in continuous polymerisations because of the changed mean residence time by loss of reactor volume. Therefore, with respect to experiments of fouling kinetics an optimal measurement point is to determine. For experiments with dynamic fouling, it is necessary to determine the point in time, when quasi stationarity of conversion is reached while fouling has not significantly reduced the reactor volume. For that purpose, the time-resolved conversion described as the proportion of formed polymer versus total monomer consumption of several reactions was observed (Fig. 2) in order to obtain the best point for in deep analytics.
Figure 2 shows that the increase in conversion is finished after two to three mean hydrodynamic residence times for the reactions. After four to five mean hydrodynamic residence times for some recipes the conversion starts decreasing because of losses of reactor volume caused by significant fouling phenomena. Concluding, the preferred spot for a comparable conversion analysis is at three mean residence times after reaction start. This is in good accordance with common literature, which summarizes that in a plug flow reactor a stationarity could be reached after three to ten residence times [38]. In this case no stationarity is reached because of the intense fouling. For further investigation this work is using the conversion after three residence times as a characteristic reaction conversion.
The experiments showed significant influences on the fouling mass for the reaction temperature (Fig. 3a), the initiator content (Fig. 3b), emulsifier content (Fig. 3c) and the monomer composition (Fig. 3d). Each data point in Fig. 3 indicates one experiment, so more data points represent replications on same conditions. The relationships between the influencing factors are complex and the strength of the influences, apparent from the change in the fouling masses, varies significantly between the parameters (Fig. 3). The influences of the parameters on the conversion are plotted, as the conversion in a tubular reactor is directly proportional to the reaction rate. Following the effects on reaction fouling can be compared to the effects on reaction rate (Fig. 3).
Temperature, initiator content and emulsifier content do not exert an independent influence on fouling mass, but rather influence the reaction conversion and determine by that the fouling mass. Fouling mass and conversion have similar time slopes. (Fig. 3a-c). Concluding this finding, fouling can be reduced by lower temperatures or less initiator but this decreases the reaction rate and conversion of the polymerisation so less product is formed. This demonstrates that it is possible to investigate the influences on fouling but regarding this parameters there is no possibility to obtain a industrially applicable recipe that avoids fouling.
An increased temperature and an increase in the initiator quantity accelerate the chemical reactions. The dependency of the conversion by emulsifier content is expected. Initiation takes place in the aqueous phase and the oligoradicals subsequently migrate into micelles or precipitate and are terminated. Consequently, an increased emulsifier content leads to a higher number of latex particles and thus to a higher yield of growing chains [39]. This causes a smaller mean particle size. (Fig. 4). Particle numbers are calculated from the particle volume for spherical particles and the total volume of the monomer fraction.
The experimentally determined mean particle sizes (Fig. 4) are decreasing with increasing emulsifier content and the resulting particle number is increasing according to literature. An acceleration of the reaction is observed.
Following the above discussion, the conversion term and the monomer composition must be considered for fouling calculations. Since the conversion is not significantly correlated with the monomer composition (R2 = 0.05 for linear correlation) the conversion X and the monomer composition, as a mole fraction of vinyl acetate xVac, are independent of each other with regard to their influence on the formation of fouling mfouling (Eq. 2).
The form and factors of these terms were obtained from 56 independent experiments (Fig. 3). For the determination of the conversion term, the fouling masses (after 120 min \({m}_{\mathrm{fouling},\mathrm{t }= 120\mathrm{ min}}\)) of the conversion-dependent process parameters are plotted against the conversion X of the reactions. The term was determined by linear fitting (Fig. 5).
In regard that only factors that influence the conversion of the reaction are changed, the fouling mass can be predicted as a function of the planned stationary conversion. Since the conversion depends on several factors the conversion term in Eq. (3) must be refined with respect to the individual conversion influences. For this purpose, the changes in the reaction conversion were determined in dependence of temperature, initiator content and emulsifier content. In order to size the influencing factors, the difference in conversion for the investigated recipe range was formed and the factors were ranked accordingly. The temperature had the strongest influence with a difference of 58% change in conversion, while emulsifier and initiator content had differences of approx. 40% change in conversion. Since the temperature has the strongest influence on the conversion, the temperature influence was determined first (Fig. 6) and the other parameters were then inserted as correction terms.
The temperature conversion term where best described by an Arrhenius-type exponential function (Eq. 4) which relates to the Arrhenius-theory [40] that the reaction rate can be described by an exponential function. If temperature T is the only change between the experiments is that the conversion X(T), which results from the reaction rate could be described temperature-dependently by an Arrhenius-type function.
For the next step, the influence of the amount of emulsifier on the reaction conversion was plotted and then integrated into the conversion term. Regarding to Koltzenburg et al. [39] or Elias [41] the reaction rate of an emulsion polymerisation dependents on the number of latices per volume [L], the monomer concentration [M], the mean number of radicals per micelle (\(\overline{n }\)), the rate coefficient (kp) and the Avogadro number (NA) (Eq. 5).
Moreover Friis and Nyhagen [42] reported an experimental correlation between the rate of polymerisation and the emulsifier content wE to the power of 0.12 for emulsion polymerisation of vinyl acetate. This finding agrees very well with the conversion dependency in this research. Due to that the description of the conversion a function, based on this equation was chosen (Eq. 6), which considers the experimental data in literature (Fig. 7) [42].
The correlation (Eq. 6) was obtained from the experimental data.
The initiator content was identified as a factor influencing the fouling mass. While the reaction rate is influenced by the initator content, the initator determines the conversion. Experimental investigations of the emulsion polymerisation of vinyl acetate by Friis and Nyhagen [42], Chang et al. [43] and Dunn and Taylor [44] are reporting correlations to the power of 0.5 to 0.6 for the reaction rate dependency of the initiator content. Besides the reaction rate the initiator content influences the ionic strength of the aqueous phase as the concentration of ions cion is increased so there might be effects that a higher ionic strength causes more fouling. This effect can’t be denied but the concentration of ionic species in the aqueous phase is only changed by the ammonium iron(III) sulphate the change in ionic concentration is below 0.201 mmol/L (Eq. 7) and should be neglectable.
The initiator content was plotted against the reaction conversion to determine the correlation (Fig. 8).
Figure 8 shows a square root function correlation (Eq. 8) and a correlation to the power of 0.56 for the initiator dependence, which are both agreeing well with the kinetics of free radical polymerisation and the experimental investigations [42,43,44]. For this model the square root function was chosen [39].
Temperature, initiator content, emulsifier content and the monomer ratio describe the fouling well when only one factor at time is changed. In order to improve the prediction flexibility, the influencing factors have to be combined in on prediction equation. Since the largest influencing factor was the temperature term, it was used at a starting point. The influences of the initiator and emulsifier content were included as correction terms. The reaction parameters T = 20 °C, wI = 1 wt%, wE = 8 wt% were used as fundamental recipe and as for the emulsifier and initiator content the relative change in conversion to this standard point was introduced in the complete conversion term (Eq. 9).
After combining Eqs. (5), (6) and (7) with Eq. (8) the following equation is obtained (Eq. 10).
By inserting the constants and summing up the coefficients, the following Eq. (11) is obtained.
With Eq. (11) the conversion of the whole reaction could be estimated and the conversion-related fouling masses mfouling could be described by combining with Eqs. (2) and (12).
Starting with Eq. (12) the coefficients were adjusted by a multivariate approach fitting all bold parameters of Eq. (12) in one step. The multivariate fitting approach agrees well to the previous described model based on single-factor evaluations as the multivariate equation shows (Eq. 13).
Regarding to Eq. (13) the multivariate fitting approach and the single-factor evaluation model agree well so both equations could be used for the description. For the following calculations the multivariate adjusted equation was used.
In addition, the influence of the comonomer composition xVAc on the fouling mass must be addressed. With increasing proportion of vinyl acetate in the polymer the fouling mass increases and can be described by a linear fit (Fig. 9) Eq. (14).
In order to improve Eq. (2) the conversion term and the monomer composition term must be combined. For that reason, the comonomer term was transformed as a change from the standard formulation with xVAc = 0.9 (Eq. 15) to describe the additional fouling expectations due to the changed comonomer ratio.
Consequently, Eq. (16) results for the entire substance system after combining all terms.
If the explicit expression for conversion is introduced into Eq. (14) the target-independent model equation (Eq. 17) is obtained.
The accuracy of the fouling prediction was determined from the scatter of the replication tests. For this purpose, seven tests were carried out under conditions that were as identical as possible and the results were compared. From these replications, the statistical component of the fouling can be estimated, as fouling processes always have a statistical range of variation. This results in an average scatter of the results of 12%, and therefore the prediction of the model also contains an uncertainty of 12%.
Comparison of calculated fouling masses with experimental data
In order to validate the model, fouling masses were determined by calculation using the model equation and then compared with experimental measured data (Fig. 10).
Experimentally measured fouling masses at t = 120 min against the model-based calculated fouling masses to validate the model. The y-error bars describe the measurement errors during the experiment, while the x-error bars indicate the intrinsic uncertainty range of the model. The solid line indicates the expected value, the dashed lines the uncertainty range of 12% given by deviation of reproduction experiments. As an example, for the red point the calculation of fouling masses is given in the supplementary information
The model predicts the fouling masses well within the uncertainties of 12% derived from the standard deviation of reproduction experiments. The calculated values and experimental fouling masses agree as well with the model. Moreover, the conversion calculated via the conversion term was compared with the measured conversion. (Fig. 11).
Experimentally measured conversions at t = 120 min against the model-based calculated conversions for specified process parameter set to validate the model. The y-error bars describe the measurement errors during the conversion determination, while the x-error bars indicate the intrinsic uncertainty range of the conversion term. As an example, the calculation of conversion for the red data point is given in the supplementary information
The description of the conversion by means of the model term corresponds approximately well with an uncertainty of ± 12% derived from the standard deviation of reproduction experiments based on the conversion value in the model.
Summary
A strategy for fouling quantification during continuous emulsion copolymerisation of vinyl acetate was established. For this strategy it is in the first step necessary to evaluate the influences of process and formulation parameters on the formation of deposits. Conversion and monomer composition were found to be relevant influencing factors. The conversion is mainly influenced by reaction temperature as well as initiator and emulsifier content. In the second step an experiment-based model was developed with which the expected fouling mass for emulsion polymerisations with similar recipes could be predicted in dependency of the influencing process and formulation parameters. Each influencing factor is described by a single term while process and formulation parameters which influence the same factor are connected via correction terms. For the investigated system the model can predict fouling masses with an accuracy of 12%. The presented strategy for developing a fouling prediction model could be used for different recipes as well so that a model can be obtained within short time.
Data availability
All raw data for the modelling process are uploaded in an word document in the Attachment area and can be published in the supplementary information as well. For further data requests the authors will be avialable.
References
Lynch J, Sutoris HF, Zubiller J, Aumüller A (1998) Verfahren Zur Polymerisation Vinylischer Monomerer. DE 196(48):811AI
Apecetche MA, Xinlai B, Cann KJ (1996) Process for reducing polymer build-up in recycle lines and heat exchangers during polymerizations employing butadiene, isoprene, and/or styrene. US 005733988A
Arhancet G (1998) Compositions and methods for inhibiting fouling of vinyl monomers. Wo 98/47593 29
Reid DK (1992) Antioxidant compositions and methods using p-phenylenediamine compounds and organic acid compounds. US 005128022A
Weber M, Lattner J, McCullough L, Dickey R, Brown S, Loezos P (2010) Olefin oligomerization reaction processes exhibiting reduced fouling. WO 2010/110801 A1
Hocking P, Sibtain F, Cheluget E (2012) Reducing fouling in heat exchangers, CA 2797489 A1
Tong DY (2015) Reducing polymer fouling and agglomeration in acrylate/methacrylate processes. US 2016/0102189 Al
Cohen L (1979) Polymerization reactors coated with polymer-inhibitor complexes. US4256864
Weimer DR, Freshour K (1979) Prevention of PVC polymer buildup in polymerization reactors using oxalyl bis(benzylidenehydrazide) and alumina. US 4145496
Wempe L, Bauman BD (1983) Method for reducing wall fouling in vinyl chloride polymerization. US 4420591
Cohen L (1987) Polymerization reactor coatings and use thereof. US 4696983
Fitzwater SJ, McFadden DM (2001) Continuous process for preparing polymers. EP 1(136):505 A1
McFadden DM, Wu RS-H (2000) The reduction of polymer fouling on reactor surfaces in a continuous process for preparing polymers. EP 1(024):149A2
Lowell JS, Hendrickson GG, Price RJ (2018) Elimination of polymer fouling in fluidized bed gas-phase fines recovery eductors. US 2018/0105613 A1
Lowell JS, Dooley KA, Li R, Aruho DK (2022) Systems and methods for mitigating polymer fouling. WO 2022/173784 Al
McDonald M, Lawrence D, Williams D (1993) Polymerization reactor. WO 93/03075
Carvalho ACSM, Chicoma DL, Sayer C, Giudici R (2010) Development of a continuous emulsion copolymerization process in a tubular reactor. Ind Eng Chem Res 49(21):10262–10273. https://doi.org/10.1021/ie100422v
Kelland MA (2020) Additives for Kinetic Hydrate Inhibitor Formulations to Avoid Polymer Fouling at High Injection Temperatures: Part 1. A Review of Possible Methods. Energy Fuels 34(3):2643–2653. https://doi.org/10.1021/acs.energyfuels.9b04040.
Dorton MR, Gardner Sr GL (2003) Process for cleaning polymeric fouling from equipment. US 20030073595A1
Haruyama H (2017) Solution conveying and cooling device. EP 3:203–177A1
Saikhwan P, Chew JYM, Paterson WR, Wilson DI (2007) Swelling and Its Suppression in the Cleaning of Polymer Fouling Layers. Ind Eng Chem Res 46(14):4846–4855. https://doi.org/10.1021/ie0615943
AlShehri A, Cunningham V, Amer A, Xu W, Melibari F (2021) Heat exchanger fouling determination using thermography combined with machine learning methods. WO 2021/026462 A1
Schork FJ, Lu Fujun F (2009) Relative Rates of Branching in Emulsion and Miniemulsion Polymerization. Macromol React Eng 3(9):539–542. https://doi.org/10.1002/mren.200900036
Schork FJ (2021) Monomer Transport in Emulsion Polymerization II: Copolymerization. Macromol React Eng 15(6):10–13. https://doi.org/10.1002/mren.202100022
Schork FJ (2022) Monomer Transport in Emulsion Polymerization III Terpolymerization and Starved-Feed Polymerization. Macromol React Eng 16(4):2–5. https://doi.org/10.1002/mren.202200010
Schork FJ (2020) Heinz Gerrens Revisited: A New Look at the Impact of Reactor Type on Polymer Chain Morphology. Macromol React Eng 14(3):1–10. https://doi.org/10.1002/mren.201900055
Schork FJ (2021) Monomer Concentration in Polymer Particles in Emulsion Polymerization. Macromol React Eng 15(3):1–2. https://doi.org/10.1002/mren.202100003
Krishnan S, Klein A, El-aasser MS, Sudol ED (2004) Effects of Agitation on Oxygen Inhibition, Particle Nucleation, Reaction Rates, and Molecular Weights in Emulsion Polymerization of n -Butyl Methacrylate. Ind Eng Chem Res 43:6331–6342. https://doi.org/10.1021/ie049796r
Deglmann P, Hellmund M, Hungenberg KD, Nieken U, Schwede C, Zander C (2019) Side Reactions in Aqueous Phase Polymerization of N-Vinyl-Pyrrolidone as Possible Source for Fouling. Macromol React Eng 13(5):1–13. https://doi.org/10.1002/mren.201900021
Neßlinger V, Welzel S, Rieker F, Meinderink D, Nieken U, Grundmeier G (2022) Thin Organic-Inorganic Anti-Fouling Hybrid-Films for Microreactor Components. Macromol React Eng 2200043:1–16. https://doi.org/10.1002/mren.202200043
Ekowati Y, Msuya M, Salinas Rodriguez SG, Veenendaal G, Schippers JC, Kennedy MD (2014) Synthetic Organic Polymer Fouling Inmunicipalwastewater Reuse Reverse Osmosis. J Water Reuse Desalin 4(3):125–136. https://doi.org/10.2166/wrd.2014.046
Hohlen A, Augustin W, Scholl S (2020) Quantification of Polymer Fouling on Heat Transfer Surfaces During Synthesis. Macromol React Eng 14(1). https://doi.org/10.1002/mren.201900035.
Hohlen A, Augustin W, Scholl S (2020) Investigation of Polymer Depositions During the Synthesis in a Heat Exchanger. Chem-Ing-Tech 92(5):629–634. https://doi.org/10.1002/cite.201900130
Gottschalk N, Kuschnerow JC, Föste H, Augustin W, Scholl S (2015) Experimental Investigation on Fouling of a Polymer Dispersion on Modified Surfaces. Chem-Ing-Tech 87(5):600–608. https://doi.org/10.1002/cite.201400126
Böttcher A, Petri J, Langhoff A, Scholl S, Augustin W, Hohlen A, Johannsmann D (2022) Fouling Pathways in Emulsion Polymerization Differentiated with a Quartz Crystal Microbalance (QCM) Integrated into the Reactor Wall. Macromol React Eng 16(2):1–8. https://doi.org/10.1002/mren.202100045
Madani M (2017) Belagsbildung in Chemischen Reaktoren Unter Berücksichtigung von Oberflächenaspekten
Bernstein C (2017) Methoden Zur Untersuchung Der Belagsbildung in Chemischen Reaktoren (Dissertation)
Hertwig K, Martens L (2010) 6 Strömungstechnisch Ideale Reaktoren Für Homogene Reaktionen. In Chemische Verfahrenstechnik. 79–183. https://doi.org/10.1524/9783486598698.79.
Klotzenburg S, Maskos M, Nuyen O (2014) Polymere; Springer Spektrum
Arrhenius S (1887) Über Die Reaktionsgeschwindigkeit Bei Der Inversion von Rohrzucker Durch Säuren. Z für Phys Chem 4U(1):226–248
Elias H-G (2002) Technische Synthesen. In Makromoleküle Set; John Wiley & Sons, Ltd. pp. 82–142. https://doi.org/10.1002/9783527626557.ch4b.
Friis N, Nyhagen L (1973) A Kinetic Study of the Emulsion Polymerization of Vinyl Acetate. J Appl Polym Sci 17(8):2311–2327. https://doi.org/10.1002/app.1973.070170802
Chang KHS, Litt MH, Nomura M (1981) The Reinvestigation of Vinyl Acetate Emulsion Polymerization (I) -The Rate of Polymerization. Appl Sci Publ Ltd I:89–136. https://doi.org/10.1007/978-94-009-8114-0_6.
Dunn AS, Taylor PA (1965) The Polymerization of Vinyl Acetate in Aqueous Solution Initiated by Potassium Persulphate at 6O °C. Die Makromol Chemie 83(1):207–0219
Funding
Open Access funding enabled and organized by Projekt DEAL. The work has been funded by the German Federal Ministry for Economic Affairs and Climate Action as part of the ENPRO Initiative (KoPPonA 2.0, FKZ: 03EN2004M).
Author information
Authors and Affiliations
Contributions
All authors conceived the experiments, Sören Rust performed the experiments and analysed the data. The first draft of the manuscript was written by Sören Rust and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest for the submitted work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rust, S., Pauer, W. Formulation and process determined fouling prediction for the continuous emulsion co polymerisation of vinyl acetate. J Polym Res 30, 242 (2023). https://doi.org/10.1007/s10965-023-03588-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-023-03588-8