Abstract
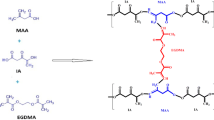
The present study was aimed for controlled delivery of capecitabine, an anticancer drug used to treat colorectal cancer. Smart pH responsive tamarind/-CD-g-poly (MAA) hydrogels were developed using the free radical polymerization process to overcome the constraints of capecitabine such as short plasma half-life, low bioavailability, and high dose frequency. The developed network system was evaluated for different characterizations including equilibrium swelling (%), drug loading efficiency (%), thermal behavior, elemental composition, morphology, complexation of components and release kinetics. Furthermore, safety profile of developed network was validated by acute oral toxicity studies and pharmacokinetic parameters were determined by carrying in-vivo studies in healthy rabbits. The grafted system proved to be thermally stable as confirmed by DSC and TGA analysis. While successful grafting, compatibility of hydrogel components and amorphous dispersion of drug within the network were shown by FTIR analysis and XRD studies. Significantly higher swelling and drug release were observed at pH 7.4 than at pH 1.2, evidencing pH responsive character of hydrogels. Maximum drug release of 94.3% was shown over period of 30 h demonstrating the controlled release profile of polymeric network. Toxicity evaluations revealed good safety profile and biocompatibility of hydrogels. Moreover, in-vivo studies showed sustained release profile of capecitabine as proved by significant increase in its half-life (13 h) and AUC (42.88 µg.h/ml) when administered as hydrogels. Hence, tamarind/β-CD-g-poly (MAA) hydrogels are strongly recommended to be employed as biocompatible pH responsive carrier system and can be utilized for controlled and targeted delivery of drugs.













Similar content being viewed by others
Data availability
The authors confirm that the data supporting the findings of this research are available within the article.
References
Li J, Mooney DJ (2016) Designing hydrogels for controlled drug delivery. Nat Rev Mater 1(12):1–17
Soni G, Yadav KS (2016) Nanogels as potential nanomedicine carrier for treatment of cancer: A mini review of the state of the art. Saudi Pharmaceutical Journal 24(2):133–139
Bhattarai N, Gunn J, Zhang M (2010) Chitosan-based hydrogels for controlled, localized drug delivery. Adv Drug Deliv Rev 62(1):83–99
Sharpe LA, Daily AM, Horava SD, Peppas NA (2014) Therapeutic applications of hydrogels in oral drug delivery. Expert Opin Drug Deliv 11(6):901–915
Mishra B, Upadhyay M, Reddy Adena S, Vasant B, Muthu M (2017) Hydrogels: an introduction to a controlled drug delivery device, synthesis and application in drug delivery and tissue engineering. Austin J Biomed Eng 4(1):1037–1049
Lu Y, Sturek M, Park K (2014) Microparticles produced by the hydrogel template method for sustained drug delivery. Int J Pharm 461(1–2):258–269
Afshar M, Dini G, Vaezifar S, Mehdikhani M, Movahedi B (2020) Preparation and characterization of sodium alginate/polyvinyl alcohol hydrogel containing drug-loaded chitosan nanoparticles as a drug delivery system. J Drug Deliv Sci Technol 56:101530
Pakizeh M, May P, Matthias M, Ulbricht M (2020) Preparation and characterization of polyzwitterionic hydrogel coated polyamide-based mixed matrix membrane for heavy metal ions removal. J Appl Polym Sci 137(48):49595
Junior CR, Fernandes RdS, de Moura MR, Aouada FA (2020) On the preparation and physicochemical properties of pH-responsive hydrogel nanocomposite based on poly (acid methacrylic)/laponite RDS. Mater Today Commun 23:100936
Jiang Q, Wang J, Tang R, Zhang D, Wang X (2016) Hypromellose succinate-crosslinked chitosan hydrogel films for potential wound dressing. Int J Biol Macromol 91:85–91
Shaw GS, Uvanesh K, Gautham S et al (2015) Development and characterization of gelatin-tamarind gum/carboxymethyl tamarind gum based phase-separated hydrogels: A comparative study. Des Monomers Polym 18(5):434–450
Nayak AK, Pal D (2017) Tamarind seed polysaccharide: an emerging excipient for pharmaceutical use. Indian J Pharm Educ Res 51:S136-146
Minhas MU, Ahmad M, Khan S, Ali L, Sohail M (2016) Synthesis and characterization of β-cyclodextrin hydrogels: crosslinked polymeric network for targeted delivery of 5-fluorouracil. Drug Deliv 9(10)
Malik NS, Ahmad M, Minhas MU (2017) Cross-linked β-cyclodextrin and carboxymethyl cellulose hydrogels for controlled drug delivery of acyclovir. PLoS ONE 12(2):e0172727
Abderaman MB, Gueye EH, Dione AN, Diouf AA, Faye O, Beye AC (2018) A molecular dynamics study on the miscibility of polyglycolide with different polymers. Int J Mater Sci Appl 7:126–132
Machackova M, Tokarsky J, Capkova P (2013) A simple molecular modeling method for the characterization of polymeric drug carriers. Eur J Pharm Sci 48:316–322
Kashirina A, Yao Y, Liu Y, Leng J (2019) Biopolymers as bone substitutes: A review. Biomater Sci 7:3961–3983
Meulenaar J, Beijnen JH, Schellens JH, Nuijen B (2013) Slow dissolution behaviour of amorphous capecitabine. Int J Pharm 441(1–2):213–217
Agnihotri SA, Aminabhavi TM (2006) Novel interpenetrating network chitosan-poly (ethylene oxide-g-acrylamide) hydrogel microspheres for the controlled release of capecitabine. Int J Pharm 324(2):103–115
Taleblou N, Sirousazar M, Hassan ZM, Khaligh SG (2020) Capecitabine-loaded anti-cancer nanocomposite hydrogel drug delivery systems: In vitro and in vivo efficacy against the 4T1 murine breast cancer cells. J Biomater Sci Polym Ed 31:72–92
Upadhyay M, Adena SK, Vardhan H, Yadav SK, Mishra B (2018) Development of biopolymers based interpenetrating polymeric network of capecitabine: a drug delivery vehicle to extend the release of the model drug. Int J Biol Macromol 115:907–919
Nandi G, Changder A, Ghosh LK (2019) Graft-copolymer of polyacrylamide-tamarind seed gum: Synthesis, characterization and evaluation of flocculating potential in peroral paracetamol suspension. Carbohyd Polym 215:213–225
García J, Ruiz-Durántez E, Valderruten N (2017) Interpenetrating polymer networks hydrogels of chitosan and poly (2-hydroxyethyl methacrylate) for controlled release of quetiapine. React Funct Polym 117:52–59
Qi X, Li J, Wei W et al (2017) Cationic Salecan-based hydrogels for release of 5-fluorouracil. RSC Adv 7(24):14337–14347
Afinjuomo F, Fouladian P, Parikh A, Barclay TG, Song Y, Garg S (2019) Preparation and characterization of oxidized inulin hydrogel for controlled drug delivery. Pharmaceutics 11(7):356
Mandal B, Ray SK (2014) Swelling, diffusion, network parameters and adsorption properties of IPN hydrogel of chitosan and acrylic copolymer. Mater Sci Eng C 44:132–143
Che Y, Li D, Liu Y et al (2016) Physically cross-linked pH-responsive chitosan-based hydrogels with enhanced mechanical performance for controlled drug delivery. RSC Adv 6(107):106035–106045
Chaves LL, Silveri A, Vieira AC et al (2019) pH-responsive chitosan based hydrogels affect the release of dapsone: design, set-up, and physicochemical characterization. Int J Biol Macromol 133:1268–1279
Mahmood T, Sarfraz RM, Akram MR, Ismail A, Qaisar MN, Shah PA (2021) Synthesis of CS-g-poly (MAA) nanogels carrier system to improve the solubility of Olmesartan Medoxomil and its in vitro evaluation. Lat Am J Pharm 40:978–990
Nesrinne S, Djamel A (2017) Synthesis, characterization and rheological behavior of pH sensitive poly (acrylamide-co-acrylic acid) hydrogels. Arab J Chem 10(4):539–547
Punyamoonwongsa P, Klayya S, Sajomsang W, Kunyanee C, Aueviriyavit S (2019) Silk sericin semi-interpenetrating network hydrogels based on PEG-Diacrylate for wound healing treatment. Int J Polym Sci
Zou C, Liu Y, Yan X, Qin Y, Wang M, Zhou L (2014) Synthesis of bridged β-cyclodextrin–polyethylene glycol and evaluation of its inhibition performance in oilfield wastewater. Mater Chem Phys 147(3):521–527
Pal P, Singh SK, Mishra S, Pandey JP, Sen G (2019) Gum ghatti based hydrogel: Microwave synthesis, characterization, 5-Fluorouracil encapsulation and ‘in vitro’drug release evaluation. Carbohyd Polym 222:114979
Hassan ZU, Bashir S, Sarfraz RM, Haroon B, Farid B, Mahmood T (2021) Comparative effectiveness of beta-cyclodextrin based copolymeric hydrogel matrices for solubility and bioavailability enhancement of lovastatin: in vitro-in vivo evaluation. LATIN Am J Pharm 40(4):822–833
Mo C-E, Chai M-H, Zhang L-P, Ran R-X, Huang Y-P, Liu Z-S (2019) Floating molecularly imprinted polymers based on liquid crystalline and polyhedral oligomeric silsesquioxanes for capecitabine sustained release. Int J Pharm 557:293–303
Rana D, Bag K, Bhattacharyya SN, Mandal BM (2000) Miscibility of poly (styrene-co-butyl acrylate) with poly (ethyl methacrylate): Existence of both UCST and LCST. J Polym Sci, Part B: Polym Phys 38:369–375
Rana D, Mandal BM, Bhattacharyya SN (1996) Analogue calorimetric studies of blends of poly (vinyl ester) s and polyacrylates. Macromolecules 29:1579–1583
Rana D, Mandal BM, Bhattacharyya SN (1996) Analogue calorimetry of polymer blends: poly (styrene-co-acrylonitrile) and poly (phenyl acrylate) or poly (vinyl benzoate). Polymer 37:2439–2443
Rana D, Mandal BM, Bhattacharyya SN (1993) Miscibility and phase diagrams of poly (phenyl acrylate) and poly (styrene-co-acrylonitrile) blends. Polymer 34:1454–1459
Roy A, Maity PP, Bose A, Dhara S, Pal S (2019) β-Cyclodextrin based pH and thermo-responsive biopolymeric hydrogel as a dual drug carrier. Materials chemistry frontiers 3(3):385–393
Alpizar-Reyes E, Carrillo-Navas H, Gallardo-Rivera R, Varela-Guerrero V, Alvarez-Ramirez J, Pérez-Alonso C (2017) Functional properties and physicochemical characteristics of tamarind (Tamarindus indica L.) seed mucilage powder as a novel hydrocolloid. J Food Eng 209:68–75
Mahmood A, Amara Sharif FM, Sarfraz RM et al (2019) Development and in vitro evaluation of (β-cyclodextrin-g-methacrylic acid)/Na+-montmorillonite nanocomposite hydrogels for controlled delivery of lovastatin. Int J Nanomed 14:5397
Sarfraz RM, Ahmad M, Mahmood A, Akram MR, Abrar A (2017) Development of β-cyclodextrin-based hydrogel microparticles for solubility enhancement of rosuvastatin: an in vitro and in vivo evaluation. Drug Des Dev Ther 11:3083
Heydari A, Pardakhti A, Sheibani H (2017) Preparation and characterization of zwitterionic poly (β-cyclodextrin-co-guanidinocitrate) hydrogels for ciprofloxacin controlled release. Macromol Mater Eng 302(6):1600501
Patil SB, Inamdar SZ, Reddy KR, Raghu AV, Soni SK, Kulkarni RV (2019) Novel biocompatible poly (acrylamide)-grafted-dextran hydrogels: Synthesis, characterization and biomedical applications. J Microbiol Methods 159:200–210
Bartil T, Bounekhel M, Cedric C, Jérôme R (2007) Swelling behavior and release properties of pH-sensitive hydrogels based on methacrylic derivatives. Acta Pharm 57(3):301–314
Xu S, Li H, Ding H et al (2019) Allylated chitosan-poly (N-isopropylacrylamide) hydrogel based on a functionalized double network for controlled drug release. Carbohyd Polym 214:8–14
Krishnat K, Dhawale SC, Remeth JD, D Havaldar V, Kavitake PR (2017) Interpenetrating networks of carboxymethyl tamarind gum and chitosan for sustained delivery of aceclofenac. Marmara Pharm J 21(4):771–782
Abdullah O, Usman Minhas M, Ahmad M, Ahmad S, Barkat K, Ahmad A (2018) Synthesis, optimization, and evaluation of polyvinyl alcohol-based hydrogels as controlled combinatorial drug delivery system for colon cancer. Adv Polym Technol 37(8):3348–3363
Kevadiya BD, Chettiar SS, Rajkumar S et al (2014) Evaluation of Montmorillonite/Poly (L-Lactide) microcomposite spheres as ambidextrous reservoirs for controlled release of Capecitabine (Xeloda) and assessment of cell cytotoxic and oxidative stress markers. Compos Sci Technol 90:193–201
Acknowledgements
The authors would like to sincerely acknowledge the “University of Sargodha and The University of Lahore, Lahore, Pakistan” for providing instrumental support in executing the present research project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rehman, U., Sarfraz, R.M., Mahmood, A. et al. Tamarind/β-CD-g-poly (MAA) pH responsive hydrogels for controlled delivery of Capecitabine: fabrication, characterization, toxicological and pharmacokinetic evaluation. J Polym Res 30, 41 (2023). https://doi.org/10.1007/s10965-022-03422-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-022-03422-7