Abstract
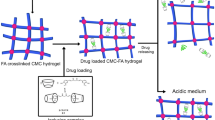
Bio-metal–organic framework (Bio-MOF) is a type of MOF which is promising in biomedical applications, including for slow-released hydrophobic drugs, for example, curcumin. In this research, we investigated the influence of surface modification of MIL-100(Fe) with carboxymethyl cellulose (CMC) and its profile in the slow-release of curcumin at different pH. MIL-100(Fe) was synthesized by the dry gel conversion (DGC) method. XRD and FTIR analysis confirmed that MIL-100(Fe) was successfully synthesized. Based on the nitrogen sorption isotherm measurement, MIL-100(Fe) is classified as micro-porous material. Morphological analysis using SEM and TEM showed that the MIL-100(Fe) formed aggregates with an average particle size of 7.37 ± 2.21 nm. Curcumin was successfully encapsulated into MIL-100(Fe) in ethanol: water with a volume ratio of 1: 1 for 48 h to produce cur@MIL-100(Fe) and has drug loading capacities of 19.267 mg/g, and drug loading efficiency up to 792.324 mg/g. Furthermore, cur@MIL-100(Fe) was modified ex-situ using CMC to form cur@MIL-100(Fe)@CMC. The release of curcumin from cur@MIL-100(Fe) and cur@MIL-100(Fe)@CMC was carried out in a 1% tween-phosphate-buffered saline (PBS) solution medium (at pH 5.8 and pH 7.4). The concentration of curcumin released was determined based on the absorbance observed on the UV–Vis spectrophotometer. Cumulative percent release (CPR) of curcumin from cur@MIL-100(Fe) at 48 h reached 82.21 ± 3.0% in tween-PBS media pH 5.8 and 40.10 ± 3.35% in tween-PBS media pH 7.4, and shows constant results afterward. The CPR of curcumin from cur@MIL-100(Fe)@CMC at 48 h reached 5.94 ± 0.02% in a tween-PBS solution of pH 5.8, and 7.64 ± 0.05% in a tween-PBS solution at pH 7.4. The presence of CMC is able to suppress the release of excessive curcumin from MIL-100(Fe) and this system shows a different release profile at different pH, so it has the potential to be used as a pH-responsive drug delivery system.









Similar content being viewed by others
Data availability
Available.
Code availability
Not applicable.
References
Qureshi M, Al-Suhaimi EA, Wahid F et al (2018) Therapeutic potential of curcumin for multiple sclerosis. Neurol Sci 39:207–214. https://doi.org/10.1007/s10072-017-3149-5
Tomeh M, Hadianamrei R, Zhao X (2019) A review of curcumin and its derivatives as anticancer agents. Int J Mol Sci 20:1033. https://doi.org/10.3390/ijms20051033
Liu W, Zhai Y, Heng X et al (2016) Oral bioavailability of curcumin: problems and advancements. J Drug Target 24:694–702. https://doi.org/10.3109/1061186X.2016.1157883
Danhier F, Ansorena E, Silva JM et al (2012) PLGA-based nanoparticles: An overview of biomedical applications. J Control Release 161:505–522. https://doi.org/10.1016/j.jconrel.2012.01.043
Anitha A, Maya S, Deepa N et al (2012) Curcumin-loaded N, O -Carboxymethyl Chitosan nanoparticles for cancer drug delivery. J Biomater Sci Polym Ed 23:1381–1400. https://doi.org/10.1163/092050611X581534
Li Y, Zheng Y, Lai X et al (2018) Biocompatible surface modification of nano-scale zeolitic imidazolate frameworks for enhanced drug delivery. RSC Adv 8:23623–23628. https://doi.org/10.1039/C8RA03616K
Ayar Z, Shafieian M, Mahmoodi N et al (2021) A rechargeable drug delivery system based on pNIPAM hydrogel for the local release of curcumin. J Appl Polym Sci 138:51167. https://doi.org/10.1002/app.51167
Saengkrit N, Saesoo S, Srinuanchai W et al (2014) Influence of curcumin-loaded cationic liposome on anticancer activity for cervical cancer therapy. Colloids Surf B Biointerfaces 114:349–356. https://doi.org/10.1016/j.colsurfb.2013.10.005
Zamani M, Rostami M, Aghajanzadeh M et al (2018) Mesoporous titanium dioxide@ zinc oxide–graphene oxide nanocarriers for colon-specific drug delivery. J Mater Sci 53:1634–1645. https://doi.org/10.1007/s10853-017-1673-6
Saputra OA, Lestari WA, Safitriono WN et al (2021) β-Amino alcohol-based organosilane tailored magnetite embedded mesoporous silica nanoparticles exhibit controlled-release of curcumin triggered by pH. Mater Lett 305:130804. https://doi.org/10.1016/j.matlet.2021.130804
Faaizatunnisa N, Lestari WW, Saputra OA et al (2022) Slow-release of curcumin induced by core-shell mesoporous silica nanoparticles (MSNs) modified MIL-100(Fe) composite. J Inorg Organomet Polym Mater 32:1744–1754. https://doi.org/10.1007/s10904-022-02230-2
Safitriono WN, Lestari WA, Wahyuningsih S et al (2022) Synthesized and release study of labelled small mesoporous silica nanoparticle as theranostic material. J Phys Conf Ser 2190:012035. https://doi.org/10.1088/1742-6596/2190/1/012035
Zhang G, Li X, Liao Q et al (2018) Water-dispersible PEG-curcumin/amine-functionalized covalent organic framework nanocomposites as smart carriers for in vivo drug delivery. Nat Commun 9:2785. https://doi.org/10.1038/s41467-018-04910-5
Charmi J, Nosrati H, Mostafavi Amjad J et al (2019) Polyethylene glycol (PEG) decorated graphene oxide nanosheets for controlled release curcumin delivery. Heliyon 5:e01466. https://doi.org/10.1016/j.heliyon.2019.e01466
Jiang J, Li Y, Liu J et al (2012) Recent advances in metal oxide-based electrode architecture design for electrochemical energy storage. Adv Mater 24:5166–5180. https://doi.org/10.1002/adma.201202146
Sun C-Y, Qin C, Wang X-L, Su Z-M (2013) Metal-organic frameworks as potential drug delivery systems. Expert Opin Drug Deliv 10:89–101. https://doi.org/10.1517/17425247.2013.741583
Abel AS, Yu Mitrofanov A, Yakushev AA et al (2019) 1,10-Phenanthroline carboxylic acids for preparation of functionalized metal-organic frameworks. Asian J Org Chem 8:2128–2142. https://doi.org/10.1002/ajoc.201900569
Wang Y, Yan J, Wen N et al (2020) Metal-organic frameworks for stimuli-responsive drug delivery. Biomaterials 230:119619. https://doi.org/10.1016/j.biomaterials.2019.119619
Rojas-Buzo S, García-García P, Corma A (2019) Zr-MOF-808@MCM-41 catalyzed phosgene-free synthesis of polyurethane precursors. Catal Sci Technol 9:146–156. https://doi.org/10.1039/C8CY02235F
Ishaq S, Tamime R, Bilad MR, Khan AL (2019) Mixed matrix membranes comprising of polysulfone and microporous Bio-MOF-1: Preparation and gas separation properties. Sep Purif Technol 210:442–451. https://doi.org/10.1016/j.seppur.2018.08.031
Efome JE, Rana D, Matsuura T, Lan CQ (2018) Insight studies on metal-organic framework nanofibrous membrane adsorption and activation for heavy metal ions removal from aqueous solution. ACS Appl Mater Interfaces 10:18619–18629. https://doi.org/10.1021/acsami.8b01454
de Lima Neto OJ, Oliveira Frós AC, Barros BS et al (2019) Rapid and efficient electrochemical synthesis of a zinc-based nano-MOF for Ibuprofen adsorption. New J Chem 43:5518–5524. https://doi.org/10.1039/C8NJ06420B
Liu Y, Ghimire P, Jaroniec M (2019) Copper benzene-1,3,5-tricarboxylate (Cu-BTC) metal-organic framework (MOF) and porous carbon composites as efficient carbon dioxide adsorbents. J Colloid Interface Sci 535:122–132. https://doi.org/10.1016/j.jcis.2018.09.086
Efome JE, Rana D, Matsuura T, Lan CQ (2019) Effects of operating parameters and coexisting ions on the efficiency of heavy metal ions removal by nano-fibrous metal-organic framework membrane filtration process. Sci Total Environ 674:355–362. https://doi.org/10.1016/j.scitotenv.2019.04.187
Tiwari A, Singh A, Garg N, Randhawa JK (2017) Curcumin encapsulated zeolitic imidazolate frameworks as stimuli responsive drug delivery system and their interaction with biomimetic environment. Sci Rep 7:12598. https://doi.org/10.1038/s41598-017-12786-6
Molavi H, Zamani M, Aghajanzadeh M et al (2018) Evaluation of UiO-66 metal organic framework as an effective sorbent for Curcumin’s overdose. Appl Organomet Chem 32:e4221. https://doi.org/10.1002/aoc.4221
Alavijeh RK, Akhbari K (2020) Biocompatible MIL-101(Fe) as a Smart Carrier with High Loading Potential and Sustained Release of Curcumin. Inorg Chem 59:3570–3578. https://doi.org/10.1021/acs.inorgchem.9b02756
Zheng Y, Rao F, Zhang M et al (2021) Efficient, selective, and reusable metal–organic framework-based adsorbent for the removal of Pb(II) and Cr(VI) heavy-metal pollutants from wastewater. Clean Eng Technol 5:100344. https://doi.org/10.1016/j.clet.2021.100344
Baati T, Njim L, Neffati F et al (2013) In depth analysis of the in vivo toxicity of nanoparticles of porous iron(iii) metal–organic frameworks. Chem Sci 4:1597. https://doi.org/10.1039/c3sc22116d
Horcajada P, Gref R, Baati T et al (2012) Metal-organic frameworks in biomedicine. Chem Rev 112:1232–1268. https://doi.org/10.1021/cr200256v
Yang X, Zhang X, Ma Y et al (2009) Superparamagnetic graphene oxide–Fe3O4 nanoparticles hybrid for controlled targeted drug carriers. J Mater Chem 19:2710. https://doi.org/10.1039/b821416f
Taherzade S, Soleimannejad J, Tarlani A (2017) Application of metal-organic framework nano-MIL-100(Fe) for sustainable release of doxycycline and tetracycline. Nanomaterials 7:215. https://doi.org/10.3390/nano7080215
Yoon JW, Seo Y-K, Hwang YK et al (2010) Controlled reducibility of a metal-organic framework with coordinatively unsaturated sites for preferential gas sorption. Angew Chem Int Ed 49:5949–5952. https://doi.org/10.1002/anie.201001230
Duan S, Li J, Liu X et al (2016) HF-free synthesis of nanoscale metal-organic framework NMIL-100(Fe) as an efficient dye adsorbent. ACS Sustain Chem Eng 4:3368–3378. https://doi.org/10.1021/acssuschemeng.6b00434
Seo Y-K, Yoon JW, Lee JS et al (2012) Large scale fluorine-free synthesis of hierarchically porous iron(III) trimesate MIL-100(Fe) with a zeolite MTN topology. Microporous Mesoporous Mater 157:137–145. https://doi.org/10.1016/j.micromeso.2012.02.027
García Márquez A, Demessence A, Platero-Prats AE et al (2012) Green microwave synthesis of MIL-100(Al, Cr, Fe) nanoparticles for thin-film elaboration. Eur J Inorg Chem 2012:5165–5174. https://doi.org/10.1002/ejic.201200710
Luo Y, Tan B, Liang X et al (2019) Dry gel conversion synthesis of hierarchical porous MIL-100(Fe) and its water vapor adsorption/desorption performance. Ind Eng Chem Res 58:7801–7807. https://doi.org/10.1021/acs.iecr.9b01647
Han L, Qi H, Zhang D et al (2017) A facile and green synthesis of MIL-100(Fe) with high-yield and its catalytic performance. New J Chem 41:13504–13509. https://doi.org/10.1039/C7NJ02975F
Bellido E, Guillevic M, Hidalgo T et al (2014) Understanding the colloidal stability of the mesoporous MIL-100(Fe) nanoparticles in physiological media. Langmuir 30:5911–5920. https://doi.org/10.1021/la5012555
Javanbakht S, Pooresmaeil M, Hashemi H, Namazi H (2018) Carboxymethylcellulose capsulated Cu-based metal-organic framework-drug nanohybrid as a pH-sensitive nanocomposite for ibuprofen oral delivery. Int J Biol Macromol 119:588–596. https://doi.org/10.1016/j.ijbiomac.2018.07.181
Karimzadeh Z, Javanbakht S, Namazi H (2018) Carboxymethylcellulose/MOF-5/Graphene oxide bio-nanocomposite as antibacterial drug nanocarrier agent. BioImpacts 9:5–13. https://doi.org/10.15171/bi.2019.02
Javanbakht S, Pooresmaeil M, Namazi H (2019) Green one-pot synthesis of carboxymethylcellulose/Zn-based metal-organic framework/graphene oxide bio-nanocomposite as a nanocarrier for drug delivery system. Carbohydr Polym 208:294–301. https://doi.org/10.1016/j.carbpol.2018.12.066
Javanbakht S, Hemmati A, Namazi H, Heydari A (2020) Carboxymethylcellulose-coated 5-fluorouracil@MOF-5 nano-hybrid as a bio-nanocomposite carrier for the anticancer oral delivery. Int J Biol Macromol 155:876–882. https://doi.org/10.1016/j.ijbiomac.2019.12.007
Siepmann J, Peppas NA (2011) Higuchi equation: Derivation, applications, use and misuse. Int J Pharm 418:6–12. https://doi.org/10.1016/j.ijpharm.2011.03.051
Freire MC, Alexandrino F, Marcelino HR et al (2017) Understanding drug release data through thermodynamic analysis. Materials 10:651. https://doi.org/10.3390/ma10060651
Unagolla JM, Jayasuriya AC (2018) Drug transport mechanisms and in vitro release kinetics of vancomycin encapsulated chitosan-alginate polyelectrolyte microparticles as a controlled drug delivery system. Eur J Pharm Sci 114:199–209. https://doi.org/10.1016/j.ejps.2017.12.012
Dehcheshmeh MA, Fathi M (2019) Production of core-shell nanofibers from zein and tragacanth for encapsulation of saffron extract. Int J Biol Macromol 122:272–279. https://doi.org/10.1016/j.ijbiomac.2018.10.176
George P, Das RK, Chowdhury P (2019) Facile microwave synthesis of Ca-BDC metal organic framework for adsorption and controlled release of Curcumin. Microporous Mesoporous Mater 281:161–171. https://doi.org/10.1016/j.micromeso.2019.02.028
Lestari WW, Arvinawati M, Martien R, Kusumaningsih T (2018) Green and facile synthesis of MOF and nano MOF containing zinc(II) and benzen 1,3,5-tri carboxylate and its study in ibuprofen slow-release. Mater Chem Phys 204:141–146. https://doi.org/10.1016/j.matchemphys.2017.10.034
Fei L, Yanhuai L, Zhongxiao S et al (2015) Rietveld study on evolution of crystalline structure of YSZ nanoparticles during co-precipitation synthesis. Rare Metal Materials and Engineering 44:2716–2720. https://doi.org/10.1016/S1875-5372(16)60025-5
Lestari WW, Hartono J, Adreane M et al (2016) Electro-Synthetic Optimization of Host Material Based on MIL-100(Fe). Molekul 11:61. https://doi.org/10.20884/1.jm.2016.11.1.195
Huang S, Yang K-L, Liu X-F et al (2017) MIL-100(Fe)-catalyzed efficient conversion of hexoses to lactic acid. RSC Adv 7:5621–5627. https://doi.org/10.1039/C6RA26469G
Mahalakshmi G, Balachandran V (2014) FT-IR and FT-Raman spectra, normal coordinate analysis and ab initio computations of Trimesic acid. Spectrochim Acta A Mol Biomol Spectrosc 124:535–547. https://doi.org/10.1016/j.saa.2014.01.061
Pangestu A, Lestari WW, Wibowo FR, Larasati L (2022) Green electro-synthesized MIL-101(Fe) and its aspirin detoxification performance compared to MOF-808. J Inorg Organomet Polym Mater 32:1828–1839. https://doi.org/10.1007/s10904-022-02235-x
Kohane DS (2007) Microparticles and nanoparticles for drug delivery. Biotechnol Bioeng 96:203–209. https://doi.org/10.1002/bit.21301
Chen G, Leng X, Luo J et al (2019) In vitro toxicity study of a porous iron(III) metal-organic framework. Molecules 24:1211. https://doi.org/10.3390/molecules24071211
Ozer D, Icten O, Altuntas-Oztas N, Zumreoglu-Karan B (2020) MIL-100(Fe) metal–organic framework catalyzed oxidation of phenol revisited: dark-Fenton activity of the catalyst. Res Chem Intermed 46:909–922. https://doi.org/10.1007/s11164-019-03997-9
Kitture R, Ghosh S, More PA et al (2015) Curcumin-loaded, self-assembled aloevera template for superior antioxidant activity and trans-membrane drug release. J Nanosci Nanotechnol 15:4039–4045. https://doi.org/10.1166/jnn.2015.10322
Acknowledgements
This work was supported by Universitas Sebelas Maret under the scheme Applied Advance Research (Penelitian Unggulan Terapan) project number 254/UN27.22/PT.01.03/2022.
Funding
This work was supported by Universitas Sebelas Maret in the research scheme of Applied Advance Research (Penelitian Unggulan Terapan) project number 254/UN27.22/PT.01.03/2022.
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions from all authors. All authors have given approval to the final version of the manuscript. Conceptualization: [Witri Wahyu Lestari], …; Methodology: [Amalia],; Formal analysis and investigation: [Amalia, Witri Wahyu Lestari, Jeesica Hermayanti Pratama, Fajar Rakhman Wibowo],; Writing—original draft preparation: [Amalia, Witri Wahyu Lestari]; Writing—review and editing: [Witri Wahyu Lestari, Larasati, Jeesica Hermayanti Pratama], …; Funding acquisition: [Witri Wahyu Lestari, Fajar Rakhman Wibowo, Teguh Endah Saraswati], …; Resources: [Witri Wahyu Lestari], …; Supervision: [Witri Wahyu Lestari, Fajar Rakhman Wibowo, Teguh Endah Saraswati].
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Conflicts of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Amalia, A., Lestari, W.W., Pratama, J.H. et al. Modification of dry-gel synthesized MIL-100(Fe) with carboxymethyl cellulose for curcumin slow-release. J Polym Res 29, 487 (2022). https://doi.org/10.1007/s10965-022-03319-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-022-03319-5